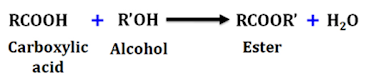Qualitative tests for Carboxylic Acids, Amide and Esters

Learning Objectives
At the end of this lecture, student will be able to
• Explain the qualitative tests for carboxylic acids
• Explain the qualitative tests for amides
• Explain the qualitative tests for esters
Qualitative tests for carboxylic acids
• Litmus test- Carboxylic acid turns blue litmus red
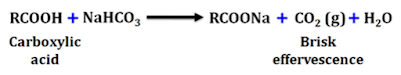
Sodium Hydrogen Carbonate Test
• Carboxylic acids reacts with sodium hydrogen carbonate to produce carbon dioxide gas which can be seen in the form of a brisk effervescence
Ester Test
• Carboxylic acid reacts with alcohol in presence of conc. sulphuric acid to form ester that is identified by the presence of a fruity smell.
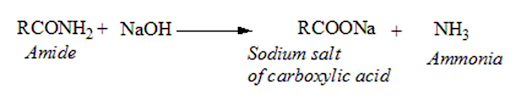
Qualitative tests for amides
• Amides are decomposed by NaOH to evolve ammonia. The gas can be tested by a moist red litmus paper which is then turned blue
Alkaline hydrolysis of aromatic amides to aromatic acid
• The soluble sodium salt of aromatic acid formed from aromatic amides upon hydrolysis is regenerated as white precipitate in acidic medium.
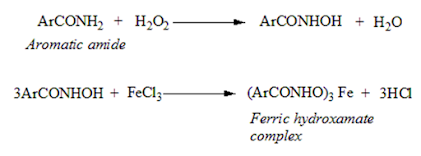
Hydroxamic acid test for aromatic primary amides:
• Hydrogen peroxide reacts with aromatic primary amides to form the hydroxamic acid, which then reacts with ferric chloride to form ferric hydroxamate complex having a violet colour.
Qualitative tests for esters
Hydroxamic acid test
• Esters react with hydroxylamines to yield hydroxamic acids which in their turn form a wine red ferric hydroxamate with ferric ions
Note
• Hydrochloric acid and acid anhydrides also form hydroxamic acids under the described conditions.
• They can be converted into salts, though, by the action of NaOH on slight warming. As salts they cannot react to hydroxamic acids
Summary
Qualitative tests for carboxylic acids
• Sodium hydrogen carbonate test
• Ester test
Qualitative tests for amide
• Alkaline hydrolysis
• Hydroxamic acid test
Qualitative tests for esters
• Hydroxamic acid test
FAQ:
Q1: What are carboxylic acids, amides, and esters? Carboxylic acids, amides, and esters are organic compounds with distinct functional groups. Carboxylic acids have the -COOH group, amides have the -CONH2 group, and esters have the -COO- group. They play vital roles in organic chemistry and various industrial applications.
Q2: Why are qualitative tests important for these compounds? Qualitative tests are essential for identifying the presence of specific functional groups in organic compounds. They help chemists confirm the identity of unknown substances and determine their chemical composition.
Q3: How can I test for the presence of a carboxylic acid? One common qualitative test for carboxylic acids is the reaction with sodium bicarbonate (baking soda). When a carboxylic acid reacts with sodium bicarbonate, it produces effervescence (bubbling) due to the release of carbon dioxide gas.
Q4: How can I test for the presence of an amide? One way to test for the presence of an amide group is to use the Ninhydrin test. When an amide is heated with Ninhydrin, it forms a purple or blue color complex, which is a positive indication of the amide functional group.
Q5: What are some common qualitative tests for esters? Esters can be identified through several tests. One common method is the silver nitrate test. When an ester reacts with silver nitrate in the presence of a base, it forms a silver mirror or a silver salt precipitate, indicating the presence of the ester group.
Q6: Can these qualitative tests differentiate between similar compounds? Yes, these tests are specific to the functional groups in carboxylic acids, amides, and esters. They can help differentiate between compounds with similar structures, ensuring accurate identification.
Q7: Are there any limitations to qualitative tests? While qualitative tests are valuable for identifying functional groups, they do not provide information about the quantity or purity of the compound. Quantitative methods are needed for such determinations.
Q8: Can these tests be used for mixtures of organic compounds? Qualitative tests can be employed on mixtures, but the results may be less straightforward. Separation techniques may be necessary to isolate individual components for accurate testing.
Q9: What is the importance of identifying these functional groups in organic chemistry? Identifying these functional groups is crucial for understanding the reactivity and properties of organic compounds. It helps chemists predict chemical reactions, synthesize new compounds, and determine the structure of unknown substances.
Q10: Where can I find detailed procedures for these qualitative tests? You can find detailed procedures for qualitative tests in standard organic chemistry laboratory manuals or textbooks. These resources provide step-by-step instructions for conducting various qualitative tests.
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy