Diuretic Drugs

Diuretic Drugs
Diuretics (“water pills”) are the drugs that increase the urine output (or) urine volume.
A chemical agent that increases the rate of urine formation.
Reabsorption of Na in the kidney results in the reabsorption of water. It follows that inhibition of Na reabsorption will result in diuresis. Because of this, the term diuretic has come to mean any agent that will inhibit the tubular absorption of sodium.
The primary mechanism of most diuretics: the direct inhibition of Na transport at one or more of the four major anatomical sites in the nephron, Because Na transport at each of these locations is unique, different rigid structural features must be possessed to inhibit Na reabsorb.
Classification of Diuretics
Diuretics can be classified by their electrolyte excretion patterns, they possess some combination of:
Natriuretic – enhanced sodium excretion
Chloruretic– enhanced chloride excretion
Saluretic – enhanced sodium chloride excretion
Kaliuretic – enhanced potassium excretion
Bicarbonaturetic – enhanced sodium bicarbonate excretion
Calciuretic – enhanced calcium excretion
Diuretics may be classified under the following two categories:
I. Mercurial diuretics
II. Nonmercurial diuretics
The nonmercurial diuretics may be classified on the basis of their chemical structure as follows:
Nephron sites of action of diuretics
Carbonic Anhydrase Inhibitors
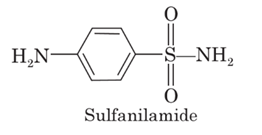
Discovered shortly after the introduction of sulphanilamide as an antibacterial.
It was observed that sulphanilamide also produced systemic acidosis and alkaline urine (HCO3− excretion).
It was shown that this activity was a result of renal carbonic anhydrase (CA) inhibition.
HETEROCYCLIC SULFONAMIDES
Structure- Activity Relationships
The prototype is Acetazolamide.
1- The sulfamoyl group is absolutely essential for the in vitro carbonic anhydrase inhibitory activity.
2- The sulfamoyl nitrogen atom must remain unsubstituted to both in vivo and in vitro activities This feature explains why all of the antibacterial sulfonamides except sulfanilamide, are incapable of inhibiting carbonic anhydrase or exerting a diuresis.
3- Substitution of a methyl group on one of acetazolamido’s ring nitrogens yields methazolamide, a product that retains carbonic anhydrase inhibitory activity & even more potent.
4- The sulfamoyl group must be attached to a moiety that possesses an aromatic character.
Methazolamide
IUPAC Name
N-(3-Methyl-5-sulfamoyl-1,3,4-thiadiazol-2(3H)-ylidene)-acetamide
Methazolamide is more potent carbonic anhydrase inhibitor than acetazolamide (the prototype), but is rarely used as a diuretic.
It is used in the treatment of glaucoma because it displays improved penetration into the eye.
Metadisulfamoylbenzene derivatives SAR
SITE 2 Diuretics, High ceiling or loop diuretics
The diuretics that belong to this class are of extremely diverse chemical structure, such as
1. The organomercurial diuretics,
2. The 5-Sulfamoyl-2- and -3-aminobenzoic acid derivatives. For example, furosemide and bumetanide respectively.
3. Phenoxyacetic acid derivatives as ethacrynic acid
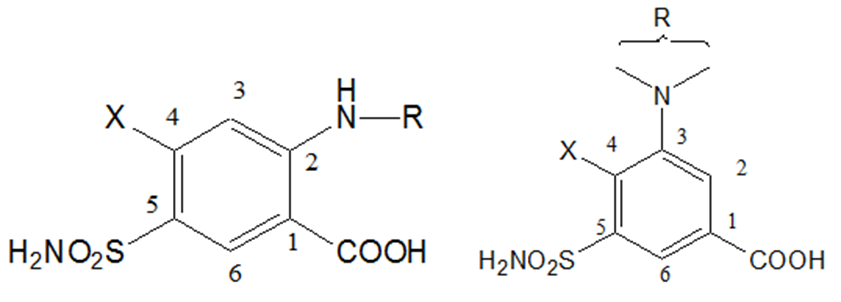
The 5-Sulfamoyl-2- and -3-aminobenzoic acid derivatives.
Uses:
Edema,
Hypertension,
Hypercalciuria (i.e., an elevated urinary concentration of calcium) are prone to the formation of calcium-containing stones within the urinary tract.
5-SULFAMOYL-3-AMINOBENZOIC ACID
SAR of 5-Sulfamoyl-2- and -3-aminobenzoic acid derivatives:
1) The substituent at the 1-position must be acidic, The carboxyl group provides optimal diuretic activity, but other groups, as tetrazole, may have respectable diuretic activity.
2) A sulfamoyl group in the 5-position is essential for optimal high-ceiling diuretic activity.
3) The activating group (x-) in the 4-position can be Cl- or CF3-, a phenoxy-, alkoxy-, anilino-, benzyl-, or benzoyl- group
1) Major differences between the two series of 5-sulfamoyl-benzoic acids in the nature of the functional groups that can be substituted into the 2-and 3-positions with the retention of maximal diuretic activity:
i) Substituent that can be tolerated on the 2-amino group of the 5-sulfamoyl-2-aminobenzoic acid series are extremely limited, and no deviations are allowed on the few moieties that are acceptable. For example, only furfural-, benzyl-, and thienylmethyl (in decreasing order) yield derivatives with maximal diuretic activity.
ii) Substituent, on the 3-amino group of the 5-sulfamoyl-3- aminobenzoic acid can very widely without affecting optimal diuretic activity.
Synthesis of Furosemide
Phenoxyacetic acids, Ethacrynic Acid, (Edecrin).
IUPAC Name
2,3-Dichloro-4-(2-methylene-1-oxobutyl)phenoxyacetic acid
Uses:
1. Same uses as cited for furosemide and bumetanide.
2. Ethacrynic acid is prescribed for individual who has a known hypersensitivity to Sulfamoyl containing drugs.
Adverse Effects:
1. Same adverse effects noted with. Furosemide and bumetanide except those related to sulfamoyl group.
2. Ototoxicity and GIT effects (GIT hemorrhage) more than furosemide and bumetanide,
Mechanism of Action: As Furosemide
SARs:
Optimal diuretic activity is achieved when:
1. An oxyacetic acid moiety is placed in the 1-position on the benzene ring,
2. A sulfhydryl-reactive acryloyl moiety is located para to the oxyacetic acid group,
3. Activating groups (Cl- or CH3-) occupy either the 3-position or the 2- and 3-positions.
4. Alkyl substituent of two- to four-carbon atoms in length occupy the position α to the carbonyl on the acryloyl moiety.
5. Hydrogen atoms occupy the terminal position of the carbon-carbon double bond of the acryloyl moiety.
Site 3 Diuretics, Thiazide and Thiazide-like Diuretics
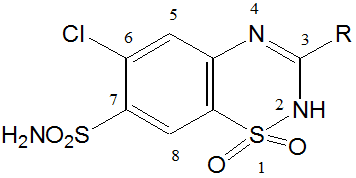
Structure-Activity Relationships Thiazide Diuretics:
1) The 2-position can tolerate small alkyl groups as CH3.
2) Substitutents in the 3-position determine the potency and duration of action of the thiazides.
3) Saturation of C-C bond between the 3 and 4 positions of the benzothiadiazine-1,1-dioxide nucleus increases the potency of this class of diuretics approximately 3-10 fold.
4) Direct substitution of the 4-, 5-, or 8-position with an alkyl group usually results in diminished diuretic activity,
5) Substitution of the 6-position with an activating group is essential for diuretic activity. The best substituent include Cl-, Br-, CF3-, and NO2– groups.
6) The sulfamoyl group in the 7-position is essential for diuretic activity.
Examples of thiazide diuretics
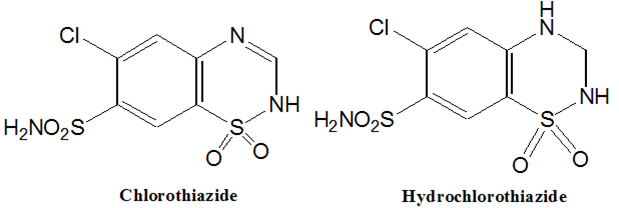
Chlorothiazide
6-Chloro-2H-1, 2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide.
Hydrochlorothiazide, (Esidrix)
6-Chloro-3, 4-dihydro-2H-1, 2,4-benzothiadiazine-7-sulfonamide 1,1 -dioxide
Synthesis of Chlorothiazide & Hydrochlorothiazide:
Site 4 Diuretics, Potassium-sparing diuretics
Diuretics that increase sodium and chloride excretion, without a concomitant increase in the urinary excretion rate of potassium. These agents are known as potassium-sparing diuretics or anti-kaliuretic agents.
Classification:
1) Aldosterone antagonists (e.g. Spironolactone)
2) Direct-acting diuretics (e.g. triamterene and amiloride)
Properties and uses:
These agents are not potent diuretics when used alone but, when combined with a thiazide – eg, Aldactizide
They reduce potassium loss, increase sodium excretion
Minimize alkalosis.
The onset of diuresis with combination therapy is much more rapid than with spironolactone alone.
Aldosterone antagonists Spironolactone
IUPAC Name
7a-(Acetylthio)-17b-hydroxy-3-oxopregn-4-ene-21-carboxylic acid g-lactone
Uses
Treatment of edema
Antihypertensive agent.
Primary use is in combination with diuretics that act at site 2 or 3 to reduce the kypokalemic effect of the latter groups of diuretics.
Osmotic diuretics:
• They have the following key features:
• 1. They are passively filtered by glomerular filtration.
• 2. They undergo limited reabsorption in the renal tubules
• 3. They are metabolically and pharmacologically inert,
• 4. They have a high degree of water solubility
• Examples, Mannitol, Theophylline
Mannitol
The prototypic osmotic diuretic,
D-Mannitol is a water-soluble, lipid-insoluble hexahydroxy alcohol. It does not diffuse GIT or renal tubule epithelium.
Mannitol should be given by the intravenous route.
Mannitol enters renal luminal fluid only by glomerular filtration. Its high luminal fluid concentration creates an osmotic effect that may prevent the reabsorption of up to 28% of the filtered load of water.
Mannitol may be employed prophylactically to avoid acute renal failure or the reduction of CSF volume and pressure.
Because solutions of mannitol may expand the extracellular fluid volume, they should not be used in patients with severe renal disease or cardiac decompensation.
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy