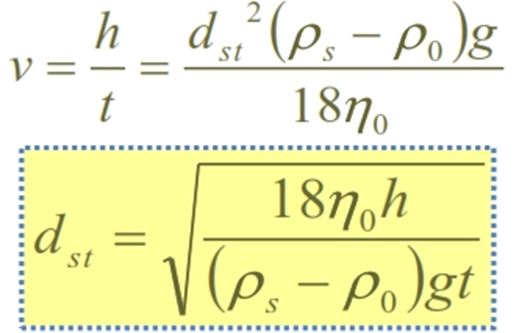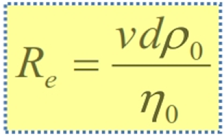Micromeritics

Learning Objectives
At the end of this lecture, student will be able to
- Explain the concept of micromeritics in pharmacy
- Discuss the applications of micromeritics in pharmaceuticals
- Define the fineness of powder according to Pharmacopoeia
- Discuss the particle size distribution and its impact on dosage forms
- Explain the concept of micromeritics in pharmacy
- Discuss the applications of micromeritics in pharmaceuticals
- Define the fineness of powder according to Pharmacopoeia
- Discuss the particle size distribution and its impact on dosage forms
- Define Edmundson’s equation
- Explain Edmundson’s equation
- Discuss the significance of Edmundson’s equation in pharmaceutical powders
- Describe the various methods of particle size determination
- Explain optical microscopy and sieve analysis methods for determining particle size
- Explain the various methods of particle size determination
- Discuss the concepts and significance of particle shape and surface area in pharmaceuticals
- Explain the methods for determination of particle surface area
- Discuss the concepts of derived properties of powders
- Explain the various derived properties of powders
- Discuss the significance of moisture content in powders and its impact on dosage form development
- Explain the flow properties of powders such as angle of repose, Carr’s index and Hausner’s ratio
- Discuss the factors influencing flow properties of powder
Micromeritics
- Definition:It is the science and technology of small particles.
- The unit of particle size is micrometer (µm), micron (µ) and I micron is equal to 10-6 m
- As particle size decreasesâ, surface area increasesá
- Micromeritics is the Science and Technology of small particles.
Knowledge and control of the size and the size range of particles are important in pharmacy because the size and surface area of a particle are related to physical, chemical and pharmacologic properties of a drug.
- The particle size of a drug can affect its release from dosage forms that are administered orally, parenterally, rectally and topically.
- In tablet and capsule manufacture, control of the particle size is essential in achieving the necessary flow properties and proper mixing of granules and powders.
PARTICLE SIZE AND SIZE DISTRIBUTION
Particle size and analysis
Sieve Number | Sieve opening |
2 | 9.5 mm |
3.5 | 5.6 mm |
4 | 4.75 mm |
8 | 2.36 mm |
10 | 2.00 mm |
20 | 850 µm |
30 | 600 µm |
40 | 425 µm |
50 | 300 µm |
60 | 250 µm |
70 | 212 µm |
80 | 180 µm |
100 | 150 µm |
120 | 125 µm |
200 | 75 µm |
230 | 63 µm |
270 | 53 µm |
325 | 45 µm |
400 | 38 µm |
PARTICLE SIZE | EXAMPLE (µm) |
0.5 to 10 | Suspension, Fine emulsion |
10 to 50 | Coarse emulsion |
50 to 100 | Fine powder |
100 to 1000 | Coarse powder |
1000 to 3350 | Average granule size |
Powders of vegetable and animal drugs are officially defined as follows:
– Very coarse (No. 8): All particles pass through a No. 8 sieve (2.36 mm) and not more than 20% through a No. 60 sieve (250 µm).
– Coarse (No. 20): All particles pass through a No. 20 sieve (850 µm) and not more than 40% through a No. 60 sieve.
– Moderately coarse (No. 40): All particles pass through a No. 40 sieve (425 µm) and not more than 40% through a No.80 sieve (180 µm).
– Fine (No. 60): All particles pass through a No. 60 sieve (250 µm) and not more than 40% through a No. 100 sieve (150 µm).
– Very fine (or a No. 80): All particles pass through a No. 80 sieve. There is no limit to greater fineness.
The powder fineness for chemicals is defined as follows
- Course (or a No. 20) powder-All particles pass through a No. 20 sieve and not more than 60% through a No. 40 sieve
- Moderately Course (or a No. 40) powder-All particles pass through a No. 40 sieve and not more than 60% through a No. 60 sieve
- Fine (or a No 80) powder-All particles pass through a No. 80 sieve. There is no limit as to greater fineness
- Very fine (or a No. 120) powder-All particles pass through a No. 120 sieve. There is no limit as to greater fineness
- Granules typically fall within the range of 4(4.75 mm) to 12- sieve size, although granulations of powders prepared in the 12- to 20-sieve (850 µm) range are sometimes used in tablet making.
- The purpose of particle size analysis in pharmacy is to obtain quantitative data on the size, distribution, and shapes of drug and nondrug components to be used in pharmaceutical formulations.
Particle Size and Size Distribution
- In a collection of particles of more than one size, two properties are important, namely.
- The shape and surface area of the individual particles.
- The particle size and size distributions (The size range and number or weight of particles).
Particle Size
- The size of a sphere is readily expressed in terms of its diameter.
- The Surface diameter, ds, is the diameter of a sphere having the same surface area as the particle.
- The Volume diameter, dv, is the diameter of a sphere having the same volume as the particle.
- The Projected diameter, dp, is the projected diameter of a sphere having the same observed area as the particle.
- The Sieve diameter, dsieve, is the diameter which describes an equivalent sphere that pass through the same sieve aperture as the asymmetric particle.
- Any collection of particles is usually Polydisperse. It is therefore necessary to know not only the size of a certain particle, but also how many particles of the same size exist in the sample
- Thus, an estimate of the size range present and the number or weight fraction of each particle size is required.
- This is the particle-size distribution and from it an average particle size for the sample can be calculated.
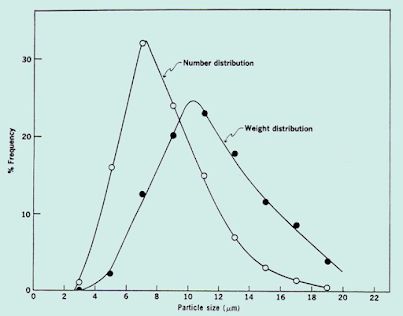
Particle Size Distribution
- When the number or weight of particles lying within a certain size range is plotted against the size range or mean particle size, a so- called Frequency distribution curve is obtained.
- This is important because it is possible to have two samples with the same average diameter but different distributions.
Applications of Micromeritics
- Release and dissolution
- Absorption and drug action
- Physical stability
- Dose uniformity
Release and dissolution
- Particle size and surface area influence the release of a drug from a dosage form.
- Higher surface area allows intimate contact of the drug with the dissolution fluids in vivo and increases the drug solubility and dissolution.
Absorption and drug action
- Particle size and surface area influence the drug absorption and subsequently the therapeutic action.
- Higher the dissolution, faster the absorption and hence quicker and greater the drug action
Physical stability
- The particle size in a formulation influences the physical stability of the suspensions and emulsions.
- Smaller the size of the particle, better the physical stability of the dosage form.
Dose uniformity
Good flow properties of granules and powders are important in the manufacturing of tablets and capsules.
Edmundson’s Equation
d mean = (Σndp+f/ Σndf)1/p
Where “n” is number of particles in each size range
“d” is the diameter of a particle in a given size range
“f” is the frequency factor
p is the index of size;
values of 1, 2 and 3 (p = 1 gives particle length, p = 2 gives particle surface and p = 3 gives particle volume)
Factor Affecting Particle size
Particle size can influence a variety of important factors, including:
1) Dissolution rate of particles intended to dissolve; drug micronization can increase the rate of drug dissolution and its bioavailability.
2) Suspendability of particles intended to remain undissolved but uniformly dispersed in a liquid vehicle (e.g., fine dispersions have particles approximately 0.5-10 µm).
3) Uniform distribution of a drug substance in a powder mixture or solid dosage form to ensure dose-to-dose content uniformity.
4) Penetrability of particles intended to be inhaled for deposition deep in the respiratory tract (e.g., 1-5 µm).
5) Lack of grittiness of solid particles in dermal ointments, creams, and ophthalmic preparations (e.g., fine powders may be 50-100µm in size).
Particle size Distribution
Size Range (Microns) | Mean Size Range | No. of particles in each range | % frequency no. of particles | % frequency weight distribution |
1-10 | 11 | 18 | 6 | 1.75 |
10-20 | 31 | 60 | 20 | 13.17 |
20-30 | 45 | 56 | 19 | 27.78 |
30-40 | 54 | 40 | 13 | 12.31 |
40-50 | 61 | 10 | 5 | 12.53 |
Particle size Distribution curves
- Two powder samples may have same mean size, but different in the size distribution above and below the mean
- Such differences are observed from frequency distribution curves
- Frequency distribution curves are the one obtained when particle size is plotted against frequency
- They are of FOUR types namely
– Frequency distribution curve Log
– Normal distribution curve
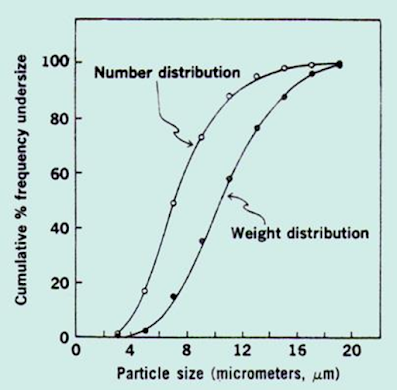
– Cumulative frequency distribution curve
– Log – probability plot
Average Particle Size
Frequency distribution curve
• The curve is Symmetrical/ Bell shaped
• Positive and negative deviations from the mean are uniform
• Not found in pharmaceutical powders
Particle Size Distribution
• Frequency distribution curve
• The curve is Asymmetric/ Skewed
• Positive and negative deviations from the mean are not uniform
• Long tail of larger particles
• Cumulative frequency distribution curve
• The curve is sigmoidal curve with the mode
• Particle size at the greatest slope
• Percentage of particles cane be found within any given size range
• But scattering of points cannot be identified
• Log-normal distribution curve
• The distribution pattern is made Symmetrical, when compared to normal distribution curve
• Powders obtained by crystallization and milling methods exhibits this pattern
• Log-probability plot
• The cumulative curve is converted into a straight line
• Slope is determined which gives geometric standard deviation
• Reference point gives geometric mean diameter
• Not used much in Pharmacy
• Number of particles per unit weight
e.g. if particle = sphere
Methods for determining particle size
• Many methods available for determining particle size such as optical microscopy, sieving, sedimentation and particle volume measurement.
1. Optical microscopy (range: 0.2-100 µm).
2. Sieving (range: 40-9500 µm).
3. Sedimentation (range: 0.08-300 µm).
4. Particle volume measurement (range: 0.5-300 µm).
5. LALLS method (Low Angle Laser Light Scattering method)
Range of particle size
A guide to range of particle sizes applicable to each method is
Particle size | Method |
1 µm | Electron microscope, ultracentrifuge, adsorption |
1 – 100 µm | Optical microscope, sedimentation, coulter counter, air permeability |
> 50 µm | Sieving |
Calibration of Eyepiece Micrometer
Each eyepiece division on the eyepiece micrometer is equivalent to
Number of divisions of stage micrometer
=———————————————————– X 10
Number of divisions of eyepiece micrometer
• Determination of particle size
• Remove stage micrometer, retaining the eyepiece micrometer on the microscope
• Place the powder sample on a clean glass slide and spread uniformly
• Place the slide under microscope and observe
• By rotating the nose piece of the microscope count and measure the length and breadth of 100 particles
• Plot a graph between class interval and cumulative frequency oversize and undersize
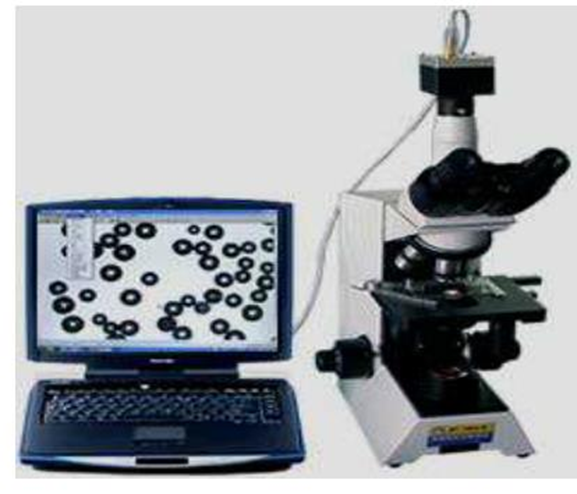
Optical microscopy
The microscope eyepiece is fitted with a micrometer by which the size of the particles may be estimated.
Optical microscopy (range: 0.2-100 µm)
• According to the optical microscopic method, an emulsion or suspension is mounted on ruled slide on a mechanical stage.
• The microscope eyepiece is fitted with a micrometer by which the size of the particles can be estimated.
• The ordinary microscope used for measurement the particle-size in the range of 0.2 to about 100 µm.
• Based on number distribution of different particle size
• Range: 0.2 – 100µm
• >200 counts (300-500 counts)
• Optical microscopy (range: 0.2 –100 um):
• The microscope eyepiece is fitted with a micrometer by which the size of the particles may be estimated
Disadvantage of microscopic method
1. The diameter is obtained from only two dimensions of the particle.
2. The number of particles that must be counted (300-500) to obtain a good estimation of the distribution makes the method somewhat slow and tedious.
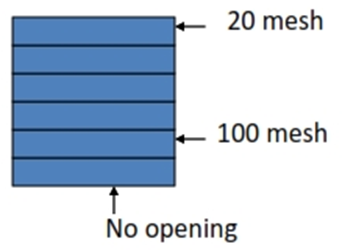
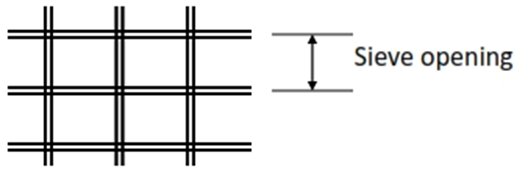
Sieve Analysis
• This method uses a series of standard sieves
• Range: 44 – 1000µm
Sieve
• Mesh number: number of openings per inch
• Sieve opening: actual size of openings between wires
Sieve number | Aperture size micrometer |
10 | 1700 |
12 | 1400 |
16 | 1000 |
22 | 710 |
30 | 500 |
36 | 425 |
44 | 325 |
60 | 250 |
85 | 36 |
100 | 35 |
120 | 34 |
150 | 32 |
For a Powder passing through Sieve no. 44 and retained on Sieve no.60, the particle size will be 325+250/2 = 287.5 microns
Sieve Analysis (Range: 40-9500 µm)
• Standard size sieves are available (IP and USP specifications) to cover a wide range of size.
• These sieves are designed to sit in a stack so that material falls through smaller and smaller meshes until it reaches a mesh which is too fine for it to pass through.
• Coarsest at top and finest at bottom
• The stack of sieves is mechanically shaken to promote the passage of the solids.
• The fraction of the material between pairs of sieve sizes is determined by weighing the residue on each sieve.
• The result achieved will depend on the duration of the agitation and the manner of the agitation.
• Particles are passed by mechanical shaking through a series of sieves of known and successively smaller size
• The determination of the proportion of powder passing through or being withheld on each sieve (range about 40-9500µm, depending upon sieve sizes).
Disadvantages
• Lower limit of particle is 40 microns
• Powder should be dry; if not will clog the mesh
• During shaking size reduction may happen; which leads to errors in estimation
Sedimentation Method
(Range: 1-200 µm)
• By measuring the terminal settling velocity of particles through a liquid medium in agravitational centrifugal environment using Andreasen appartus.
• The diameter is obtained by gravity sedimentation
• Prepare 2% suspension of light magnesium oxide containing 0.2%w/v polyvinyl pyrollidone as deflocculating agent
• Pour the suspension into Andreasen pipette and make up the volume to 550ml with purified water
• Note the height of suspension in Andreasen pipette
• Pipette out 10ml of sample from Andreasen pipette and transfer to a tarred china dish
• Evaporate the contents of china dish till the residue is obtained
• Weigh china dish and note the weight
• The difference in weight will give the fraction of solid collected at given time interval
• Repeat the process at the interval of every 5 minutes and continue up to 30 minutes
Sedimentation
Stoke’s law
Where
v: rate of settling
h: distance of fall in time t
rs: density of particle
r0: density of dispersion medium
g: acceleration due to gravity
h0: viscosity of medium
Reynolds number Re
• Re > 0.2 à Stoke’s law cannot be used
• Flow should be laminar and not turbulent
• Suspension should be dilute (1 -2%)
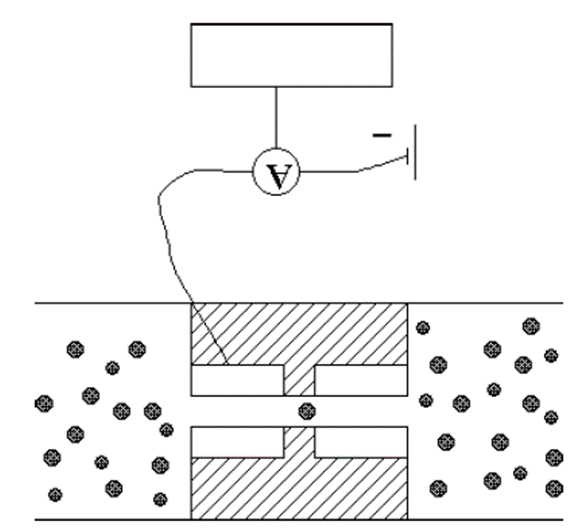
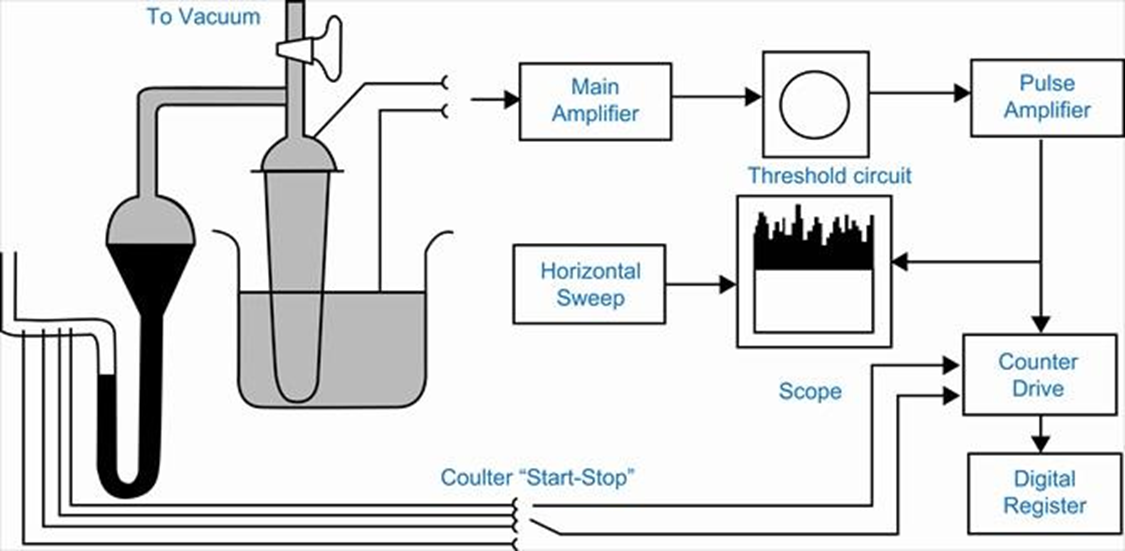
Particle volume measurement – Coulter Counter
(Range: 0.5-300 µm)
• In this type the powder is suspended in an electrolyte solution
• This suspension is then made to flow through a short insulated capillary section between two electrodes and the resistance of the system is measured.
• When a particle passes through the capillary there is a momentary peak in the resistance, the amplitude of the peak is proportional to the particle size.
• Counting is done by a computer.
Coulter Counter
LALLS METHOD
• LALLS – Low Angle Lase Light Scattering Technique
PARTICLE SHAPE AND SURFACE AREA
Specific Surface
• The surface area per unit weight (Sw)
d = diameter of particles
Ρ = density of powder
METHODS FOR DETERMINING SURFACE AREA
Adsorption Method
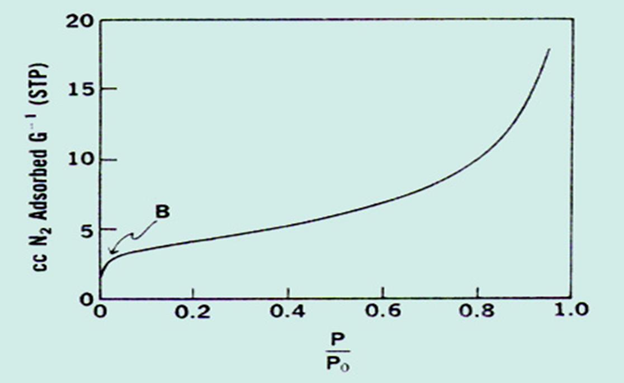
• The volume in cubic centimeters of gas adsorbed per gram of adsorbent may be plotted against the pressure of the gas at constant temperature
• Using Solute
Powder sample + Methanolic solution of magnesium stearate —– Mix for 1 hour —–Filter —– Filtrate contains unadsorbed stearic acid —- Estimate this by titrating with sodium hydroxide — Calculate the adsorbed stearic acid/gm of powder using Avagadro’s number (6.023X1023), by which no.of molecules/gm can be calculated —– If area occupied by one molecule is known, then the total surface area occupied by all molecules can be calculated which gives the Total Surface Area of the given powder sample
• Using Adsorption of Gas – QUANTASORB
Powder sample placed in a cell —– Pass Nitrogen and Helium gas —– Amount of nitrogen adsorbed at every equilibrium pressure was measured using thermal conductivity detector ——- BELL shaped curve will be obtained —— Signal height gives the rate of adsorption of nitrogen gas and AUC give amount of nitrogen gas adsorbed in 1cm3 on powder sample using BET equation
P 1 (b-1)P
——— = —— + ———-
V(P0-P) Vmb VmbP0
• V = volume of gas in 1cm3 adsorbed/gm of powder at pressure P
• P0 = saturated vapour pressure of nitrogen gas
• b = constant that gives difference between Heat of adsorption and Heat
Air Permeability Method
• The principle resistance to the flow of a fluid, such as air, through a plug of compressed powder is the surface area of the powder
• The flow rate through the plug, or bed, is affected by
– The degree of compression of the particles
– The irregularity of the capillaries
FISHER SUB SIEVE SIZER
• Based on Porosity
• As porosity decreases, surface area powder also increases
• When air is passed through the plug, resistance to flow occurs
• This resistance is related to surface area, which is determined by Kozency-Carman equation
v= A Ù pt Ɛ3/ƞS2w.
Kl. (1-Ɛ)2
A- C.S area of plug s- specific surface
K – Constant ( 5±0.5) v – volume of air that flowed in time‘t’
ƞ – viscosity of air
Ɛ – Porosity t – time of flow in secs
Ùp – pressure difference, l = length of sample holder
Derived Properties of Powders
• Porosity
• Packing arrangement
• Densities of particles
• Bulkiness
• Flow properties
• Compaction
Porosity (e)
• Void volume (v): the volume of space
• Bulk volume (Vb) : occupied volume
• True volume (Vp)
• Porosity = Bulk volume – True volume / Bulk volume (OR)
• Porosity = True density – Bulk density / True density
• Pharmaceutical powders should possess 30-50% porosity
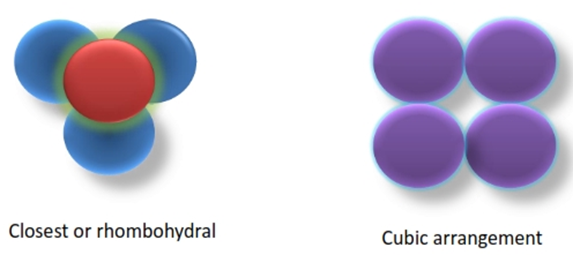
Packing Arrangements
• Two ideal packing materials
1. Closest or rhombohydral
2. Most open, loosest or cubic packing.
• Theoretical porosity of powder consist of uniform sphere in
• Closest packing- 26%
• Loosest packing- 48%
• Real powder have porosity in between 30 to 50%.
• In suspension, porosity may above the theoretical max limit 48%.
• Crystalline materials porosity- <1% (under force 10000 lb/in2)
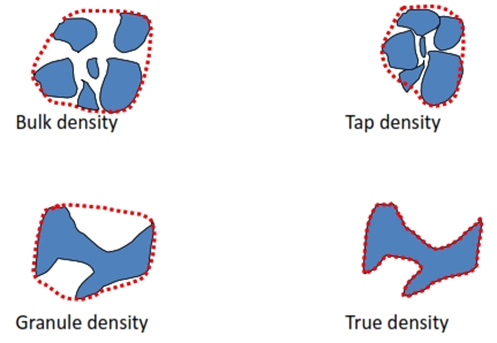
Densities of Particles
True density (r)
• Density of the actual solid material. Ratio of given mass of powder and its true volume
• True volume = Bulk volume – Void volume
• Determined by Liquid displacement method
Granule density (rg) (Particle density)
•The mass of particles divided by the volume as determined by the Mercury displacement method
True density measurements-
• For non-porous solid
– True density & granule density identical.
– Both obtained by-
– Helium displacement method
– Liquid displacement method
• For porous material (having an internal surface)
– Using helium densitometer
Bulk density
Bulk density = mass of the powder (w) / bulk volume (Vb)
• When particle are loosely packed, lots of gaps in between particle.
• Bulk volume increases making powder light.
• Powder classified as ‘light’or ‘heavy’ “light powder have high bulk volume”
• ‘Bulk density apparatus’ is used to determine bulk volume.
Applications:-
• Used to check uniformity of bulk chemicals.
• Size of capsule determine by bulk volume.
• Higher the bulk volume bigger the size of capsule.
Densities of Particles
• During tapping, particles gradually pack more efficiently, the powder volume decreases and the tapped density increases.
Moisture Content
• Higher the moisture content greater the cohesion & adhesion.
• Flow properties can be improved by following methods- Powder processes into granules to improve flow.
• Choosing optimum size of granules (400 to 800 um)
• Incorporating optimum amount of fines (about 15%)
• Incorporating optimum concentration of lubricants (magnesium stearate, talc)
Bulkiness
• Bulkiness (bulk) is specific bulk volume, the reciprocal of bulk density
• It is an important consideration in the packaging of powders.
• The bulk density of calcium carbonate vary from 0.1 to 1.3, and the lightest (bulkiest) type require a container about 13 times larger than that needed for the heaviest variety.
• Bulkiness increases with a decrease in particle size
• In mixture of materials of different sizes, the smaller particles sift between the larger ones and tend to reduce bulkiness.
Light vs. Heavy Powders
• Light: low bulk density or large bulk volume
• Heavy: high bulk density or small bulk volume
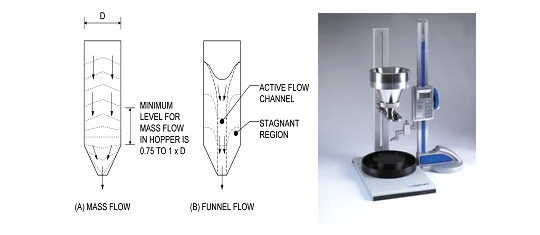
Flow Properties
• A bulk powder is somewhat analogous to a non-Newtonian liquid (plastic flow, dilatancy)
• Flow property is affected by particle size, shape, porosity, density, surface texture
• Measurement: angle of repose (f) (= f(roughness))
tan f = m
• m: coefficient of friction
Non-uniformity (segregation) in blending
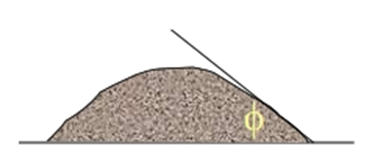
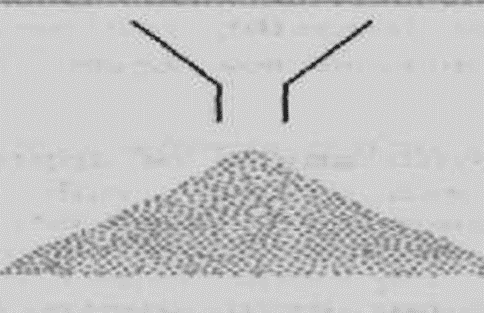
Angle of repose
• The angle of repose is a relatively simple technique for estimating the flow properties of a powder.
• It can easily be determined by allowing a powder to flow through a funnel and fall freely onto a surface
• The height and diameter of the resulting cone are measured and the angle of repose calculated from this equation:
tanf=h/r
Where
h is the height of the powder cone
r is the radius of the powder cone.
• The sample is poured onto a horizontal surface and the angle of the resulting pyramid is measured.
The user normally selects the funnel orifice through which the powder flows slowly and reasonably constantly.
• Angle of repose less than 20 (excellent flow)
• Angle of repose between 20-30 (good flow)
• Angle of repose between 30-34 (Pass flow)
• Angle of repose greater than 40 (poor flow)
• The rougher and more irregular the surface of the particles, the higher will be the angle of repose.
Porosity, void, and bulk volume
• The characteristics used to describe powders include porosity, true volume, bulk volume, apparent density, true density, and bulkiness.
Porosity is void x 100%
• This value should be determined experimentally by measuring the volume occupied by a selected weight of a powder, Vbulk.
• The true volume, V, of a powder is the space occupied by the powder exclusive of spaces greater than the intramolecular space.
Void can be defined as
(Vbulk-V)/Vbulk
• Therefore, porosity is (Vbulk-V)/Vbulkx100%
• The bulk volume is true volume + porosity
Apparent density (Bulk), true density, and bulkiness
• The apparent (bulk) density, ra, is
• Weight of the sample/Vbulk
• The true density, r, is
• Weight of the sample/V
• The bulkiness, B, is the reciprocal of the apparent density,
B=1/ ra
Carr’s compressibility index
• A volume of powder is filled into a graduated glass cylinder and repeatedly tapped for a known duration. The volume of powder after tapping is measured.
• Carr’s index (%) = Tapped density – Poured or bulk density x 100
Tapped density
• Bulk density = weight / bulk volume
• Tapped density = weight / true volume
Relationship between powder flowability and % compressibility
Flow | % |
Excellent flow | 5 – 15 |
Good | 16 – 18 |
Fair | 19 – 21 |
Poor | 22 – 35 |
Very poor | 36 – 40 |
Extremely poor | > 40 |
Hausner ratio
Hausner ratio = Tapped density / Poured or bulk density
• Hausner ratio was related to interparticle friction:
• Value less than 1.25 indicates good flow (= 20% Carr).
• The powder with low interparticle friction, such as coarse spheres.
• Value greater than 1.5 indicates poor flow (= 33% Carr).
• More cohesive, less free-flowing powders such as flakes.
• Between 1.25 and 1.5, added glidant normally improves flow.
• > 1.5 added glidant doesn’t improve flow.
Dispersibility
• It is the ability of a material to flow or pour easily over a planes.
• Dispersability, dustiness, & floodability are inter- related term.
weight of powder in watch glass
Dispersability (%) = ———————————————-ˣ 100
initial weight of the sample
Dispersibility apparatus:-
• A hallow cylinder through which is Drop from a height 61 cm above the glass watch.
Compression Properties
• This property normally used for the preparation of the tablet.
• This process also called compaction.
• During this porosity of powder changes.
• Plastic behaviour:-
– Deformed on compression
– Compact powder get deformed which is tapped into close packing.
– For e.g. kaolin which have soft & spongy particle
• Dilatant behaviour:-
– Shows unexpected expansion under the stress.
– Some substances when compacted exhibits higher porosity than the powder in close packing.
• For e.g. sodium chloride.
• Compression properties of most drugs extremely poor.
• Hence compression vehicle is added such as-
1) Lactose
2) Calcium phosphate
3) Microcrystalline cellulose
• Low dose drug tablet prepared by direct compression
• But high dose drug prepared by granulation methods.
• Tablet material should be plastic i.e. undergoing permanent deformation
Flow Through Orifice Powder flow meter
Factors affecting the flow properties of powder
1. Alteration of Particle’s size & Distribution
2. Alteration of Particle shape & texture
3. Alteration of Surface Forces
4. Formulation additives (Flow activators)
Alteration of Particle’s size & Distribution
• There is certain particle size at which powder’s flow ability is optimum.
• Coarse particles are more preferred than fine ones as they are less cohesive.
• The size distribution can also be altered to improve flowability by removing a proportion of the fine particle fraction or by increasing the proportion of coarser particles such as occurs in granulation.
Alteration of Particle shape & texture
Particle’s Shape
• Generally, more spherical particles have better flow properties than more irregular particles.
• Spherical particles are obtained by spray drying, or by temperature cycling crystallization.
Particle’s texture
• Particles with very rough surfaces will be more cohesive and have a greater tendency to interlock than smooth surfaced particles.
Alteration of Surface Forces
• Reduction of electrostatic charges can improve powder flowability.
• Electrostatic charges can be reduced by altering process conditions to reduce frictional contacts.
• Moisture content of particle greatly affects powder’s flowability.
• Adsorbed surface moisture films tend to increase bulk density and reduce porosity.
• Drying the particles will reduce the cohesiveness and improve the flow.
• Hygroscopic powder’s stored and processed under low humidity conditions.
Formulation additives (Flow activators)
• Flow activators are commonly referred as a glidants.
• Flow activators improve the flowability of powders by reducing adhesion and cohesion.
e. g. Talc, maize starch and magnesium stearate.
METHODS OF IMPROVING FLOW PROPERTIES
• Increasing the average particle size
• By producing the powder in the form of spherical particles
• By use of additives
INCREASING THE AVERAGE PARTICLE SIZE
• The larger particles are less cohesive than smaller ones and the optimum size for free flow exists and also a distinct disadvantages in using a finer grade is noted.
• Hence granules are used in many cases than powder forms and also the addition of coarser fraction to fine powder improves its flow property.
BY PRODUCING THE POWDER IN THE FORM OF SPHERICAL PARTICLES
• By using this type of powder, it packs down and flows easily since particles roll over one another.
BY USE OF ADDITIVES
• Commonly used additives to increase the flow properties of the powders are flow enhancers
• They are generally used in low conc’s and the optimum conc of all glidants varies from 1% to 2% and above this conc, the flow properties of powders decreases.
Various mechanisms of glidant action
• Dispersion of static charge from surface of host particles
• Distribution of glidants in host particles
• Adsorption of gases and vapours are adsorbed onto host particles
• Physical separation of particles and reduction in vanderwaal’s interaction.
• Adsorption of glidant particles to granulation surfaces so that friction between particles and surfaces are minimized.
• The effect of glidant depends on may factors such as…
1, Physical & chemical affinity of powder
2, Average particle size & shape
3, Concentration of glidant
4, Degree of mixing
5, Moisture content
• Glidants generally has mechanical action …they adhere to surface of host powders and reducing their tendency to interlock mechanically during movement and flow.
• In general 100µm powder requires about 3% of 1 µm glidant
Summary
• Micromeritics is defined as the science and technology of small particles.
• The unit of particle size used in the micrometer (µm), micron (µ) and equal to 10-6 m.
• The particle size of a drug can affect its release from dosage forms that are administered orally, parenterally, rectally and topically
• Particle size is expressed as Surface diameter, Volume diameter, projected diameter and Stokes diameter.
• Micromeritics has applications in drug release and dissolution, absorption and drug action, Physical stability and dose uniformity.
• d mean = (Σndp+f/ Σndf)1/p – Edmundson’s Equation
• Particle size distribution curves including
– Frequency distribution curve
– Cumulative percent curve
– Log normal distribution curve
– Log probability curve
• Various methods of particle size determination including
– Optical microscopy (range: 0.2-100 µm).
– Sieving (range: 40-9500 µm).
– Sedimentation (range: 0.08-300 µm).
– Particle volume measurement (range: 0.5-300 µm).
– LALLS method (Low Angle Laser Light Scattering method)
• Particle size can be determined by Sedimentation, Particle volume measurement and LALLS method (Low Angle Laser Light Scattering method)
• Specific surface area is determined by Air permeability and Adsorption method
• Fischer Sub sieve sizer is used to determine particle surface area
• Various derived properties of pharmaceutical powders include Porosity, Packing arrangement, Densities of particles Bulkiness, Flow properties and Compaction
• Role of angle of repose, Carr’s index and Hausner’s ratio has been discussed with emphasis on dosage form development
• Significance of moisture content in powders and its impact on dosage form development was discussed
• Factors that influence pharmaceutical powders are Alteration of Particle’s size & Distribution, Alteration of Particle shape & texture, Alteration of Surface Forces and Formulation additives (Flow activators)
• Flow properties can be enhanced by increasing the average particle size, by producing the powder in the form of spherical particles and by use of additives
Also, Visit: Biotechnology Notes