Suspension

Learning Objectives
At the end of this lecture, student will be able to
• Explain the concepts of coarse dispersion
• Discuss the reasons for formulating suspensions
• List the ideal requirements of a suspension
• Classify the types of suspension
• Distinguish between flocculated and deflocculated suspension
• Discuss the sedimentation rate of suspension
• Explain the desirable features of the dispersed phase of a suspension
• Explain the concepts of formulation of suspension
• Discuss the rate of settling and aggregation of particles in a suspension
• Discuss the electrical properties of suspension
• Explain DLVO theory
• Discuss the controlled flocculation and rheology of suspension
• Explain the methods of evaluation of suspension
Suspensions
• Suspension is defined as preparations containing finely divided drug particles distributed somewhat uniformly throughout a vehicle in which the drug exhibits a minimum degree of solubility.
• Some suspensions are available in ready-to-use form that is, already distributed through a liquid vehicle with or without stabilizers and other additives.
• Other preparations are available as dry powders intended for suspension in liquid vehicles.
• This type of product generally is a powder mixture containing the drug and suitable suspending and dispersing agents to be diluted and agitated with a specified quantity of vehicle, generally purified water.
Reasons for Use of Suspension
• Drug is insoluble
• Drug is more stable in suspension or emulsion
• There is a need to control the rate of release of the drug
• Drug has bad taste (oral)
Reasons for Suspensions
There are several reasons for preparing suspensions.
1)The suspension ensures chemical stability while permitting liquid therapy.
2)For many patients, the liquid form is preferred to the solid form of the same drug because of the ease of swallowing liquids and the flexibility in administration of a range of doses
3)The disadvantage of a disagreeable taste of certain drugs in solution form is overcome when the drug is administered as undissolved particles of an oral suspension.
Features Desired in a Pharmaceutical Suspension
There are many considerations in the development and preparation of a pharmaceutically elegant suspension.
1. A properly prepared pharmaceutical suspension should settle slowly and should be readily redispersed upon gentle shaking of the container.
2. The particle size of the suspension should remain fairly constant throughout long periods of undisturbed standing.
3. The suspension should pour readily and evenly from its container.
Routes of Administration
• Oral
• Ocular
• Otic
• Rectal
• Parenteral
• Topical
Oral Suspensions
• For elderly, children etc., liquid drug form is easier to swallow
• Liquid form gives flexibility in dose range
• Majority are aqueous with the vehicle flavored and sweetened.
• supplies insoluble, distasteful substance in form that is pleasant to taste
Examples
• Antacids, tetracycline HCl, indomethacin
Topical Suspension (Lotions)
• Most often are aqueous
• Intended to dry on skin after application (thin coat of medicianl component on skin surface)
• Label stating “to be shaken before use” and “for external use only”
Examples:
– Calamine lotion
– Hydrocortisone 1 – 2.5 %
– Betamethasone 0.1%
Ophthalmic Suspension
• used to increase corneal contact time (provide a more sustained action)
Intramuscular Suspension
• Formation of drug depots (sustained action)
Examples:
• Procaine penicillin G
• Insulin Zinc Suspension
• Addition of ZnCl2
• Suspended particles consist of a mixture of crystalline and amorphous zinc insulin (intermediate action)
• Extended Insulin Zinc Suspension
• Solely zinc insulin crystals à longer action
• Contraceptive steroids
Disadvantages of Suspension
• Uniformity and accuracy of dose – not as good as tablet or capsule
• Adequate particle dispersion
• Sedimentation, cake formation
• Product is liquid and bulky
• Formulation of an effective suspension is more difficult than for tablet or capsule
Requirements of an ideal suspension
• No rapid settling
• If they settle, should not form a hard cake at the bottom
• Easily redispersible
• Easily pourable
• Flow through syringe and needle
• Color and odor should be acceptable
• Spread easily on the surface of the skin
Deference between Deflocculated Suspension and Flocculated Suspension
Deflocculated Suspension | Flocculated Suspension |
Individual particles | Separate particles |
Rate of sedimentation is low | Rate of sedimentation is high |
Less sediment volume | More sediment volume |
Pleasing appearance | Unpleasing appearance |
More bioavailabilty | Less bioavailabilty |
Redistribution on shaking is difficult | Redistribution on shaking is easy |
Sediment closely packed | Sediment cloosely packed |
Supernatant is cloudy | Supernatant is clear |
Applications of Suspension
• Provides better stability – When drugs are not stable in solution form, an insoluble form of drug so that it doe not degrade. Eg. Procaine Penicillin G
• Choice of solvent – When solvent other than water is not acceptable suspension is the only choice. Eg. Corticosteroids
• Taste masking – of bitter drugs Eg. Roxithromycin
• Sustained action of drug – Eg. Dextromethorphan Hydrobromide
• Improved bioavailability – Eg. Aluminium hydroxide suspension
Sedimentation Rate of the Particles of a Suspension
Stokes’ Equation
dx/dt=d2(ρi–ρe)g/18h
18ƞh
dst = ———-
(ρs-ρl)gt
Where
– dx/dt is the rate of setting
– d is the diameter of the particles,
– ρi is the density of the particle,
– ρe is the density of the medium,
– g is the gravitational constant,
– h is the viscosity of the medium
•A number of factors can be adjusted to enhance the physical stability of a suspension, including
– Diameter of the particles
– Density of particles
– Viscosity of the medium
• Stokes’ equation does not apply precisely to the usual pharmaceutical suspension that is irregularly shaped and of various particle diameters.
• The fall of the particles does result in both turbulence and collision, and also in which the particles may have some affinity for the suspension medium.
Physical Features of the Dispersed Phase of a Suspension
• The most important consideration in suspensions is size of the particles.
• In most pharmaceutical suspensions, the particle diameter is 1 to 50 mm.
• Particle size reduction is generally accomplished by dry milling prior to incorporation of the dispersed phase into the dispersion medium.
• One of the most rapid, convenient, and inexpensive methods of producing fine drug powders of about 10 to 50 mm size is micropulverization.
• For still finer particles, under 10 mm, fluid energy grinding (jet milling or micronizing), is quite effective.
• Particles of extremely small dimensions may also be produced by spray-drying.
• The reduction in the particle size of a suspensoid is beneficial to the stability of the suspension.
• However, reducing the particle size too much should be avoided, since fine particles have a tendency to form a compact cake upon settling to the bottom of the container.
• To avoid formation of a cake, it is necessary to prevent agglomeration of the particles into larger crystals or into masses.
• One common method of preventing rigid cohesion of small particles of a suspension is intentional formation of a less rigid or loose aggregation of the particles held together by comparatively weak particle-to-particle bonds.
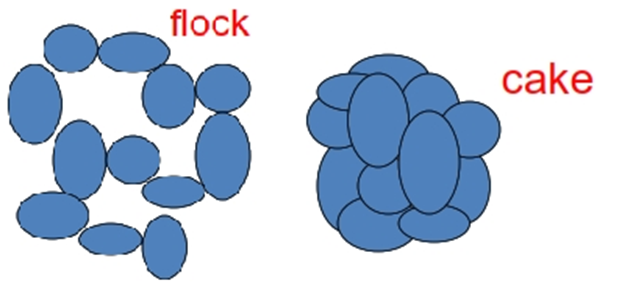
• Such an aggregation of particles is termed a floc or a floccule The flocs settle to form
• A higher sediment volume than unflocculated particles, the loose structure of which permits
• The aggregates to break up easily
• Distribute readily with a small amount of agitation.
There are several methods of preparing flocculated suspensions,
• For an oral suspension of drug, clays such as diluted bentonite magma are commonly employed.
• In a parenteral suspension, frequently a floc of the dispersed phase can be produced by an alteration in the pH of the preparation.
• Electrolytes can also act as flocculating agents.
• The carefully determined concentration of surfactants can also induce flocculation of particles in suspension and increase the sedimentation volume.
Dispersion medium
• Oftentimes, as with highly flocculated suspensions, the particles of a suspension settle too rapidly to be consistent with what might be termed a pharmaceutically elegant preparation.
• In many commercial suspensions, suspending agents are added to the dispersion medium to lend it structure.
The following suspending agents are commonly used
• Carboxymethylcellulose
• Methylcellulose
• Microcrystalline cellulose
• Polyvinyl pyrrolidone
• Xanthan gum
• Bentonite
Well Formulated Suspension
• Resuspend easily upon shaking
• Settle rapidly after shaking
• Homogeneous mix of drug
• Physically and chemically stable during its shelf life
• Sterile (parenteral, ocular)
• Gets into syringe (parenteral, ocular)
“External” Forces Acting on Particles
• In the preparation of a suspension, the characteristics of both the intended dispersed phase and the dispersion medium is important.
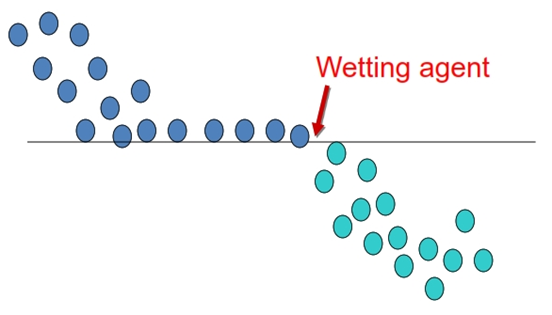
• Some drugs are not penetrated easily by the vehicle and have a tendency to clump together or to float on top of the vehicle.
• In this case, the powder must first be wetted by a “wetting agent” to make the powder more penetrable by the dispersion medium.
• Alcohol, glycerin, and other hygroscopic liquids are employed as wetting agents when an aqueous vehicle is to be used as the dispersion phase.
Formulation of Suspensions
Wetting
• In the large-scale preparation of suspensions the wetting agents are mixed with the particles by an apparatus such as a colloid mill.
• On a small scale, they are mixed with a mortar and pestle.
• Once the powder is wetted, the dispersion medium is addedin portions to the powder, and the mixture is thoroughly blended before subsequent additions of vehicle.
• A portion of the vehicle is used to wash the mixing equipment free of suspensoid, and this portion is used to bring the suspension contains the desired concentration of solid matter.
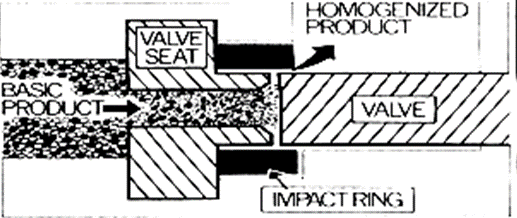
• The final product is then passed through a colloid mill or final product is then passed through a colloid mill or other blender or mixing device to insure uniformity
Settling and Aggregation
• The suspension shall form loose networks of flocks that settle rapidly, do not form cakes and are easy to resuspend.
• Settling and aggregation may result in formation of cakes (suspension) that is difficult to resuspend or phase separation (emulsion)
Factors influencing settling
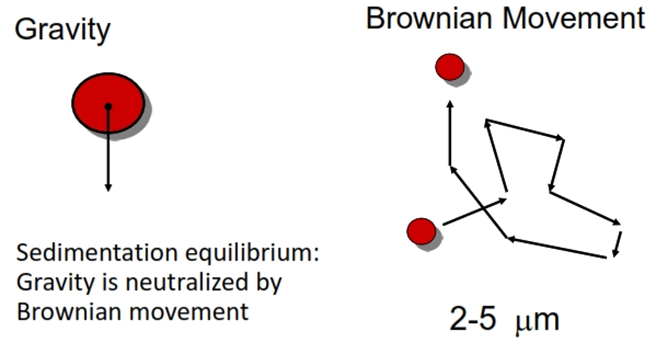
Brownian motion
• Based on size of particles (upto 5micron size)
• Also it depends on viscosity and density of medium
• Brownian motion prevents sedimentation
• If particle size is reduced to half of the original size, rate of sedimentation decreases by four times
• Viscosity should be optimum, higher the viscosity, lower the sedimentation rate
• High viscosity increases physical stability – due to retardation of sedimentation
• High viscosity inhibits crystal growth – due to restriction of movement of particles
• High viscosity prevents the transformation of metastable crystals to stable crystals
• But high viscosity reduces drug absorption, problems in pourability and reduces redispersibility of suspension
• When density of medium = density of solids, rate of settling will be ZERO
• If the density of medium is increased, the difference in densities will be less
Sedimentation parameters
Eventually Ff = Fb and reach terminal velocity
Stokes’ Law
Where
v = terminal velocity (cm/s)
d = diameter (cm)
ρs = density of dispersed phase
ρo = density of continuous phase
ƞo = viscosity of continuous phase (Pa s)
Physical Stability
• The large surface area of dispersed particles results in high surface free energy DG = ƳSL DA
• Thermodynamically unstable
• Can reduce ƳSL by using surfactants but not often can one reach DG = 0
• Particles tend to come together
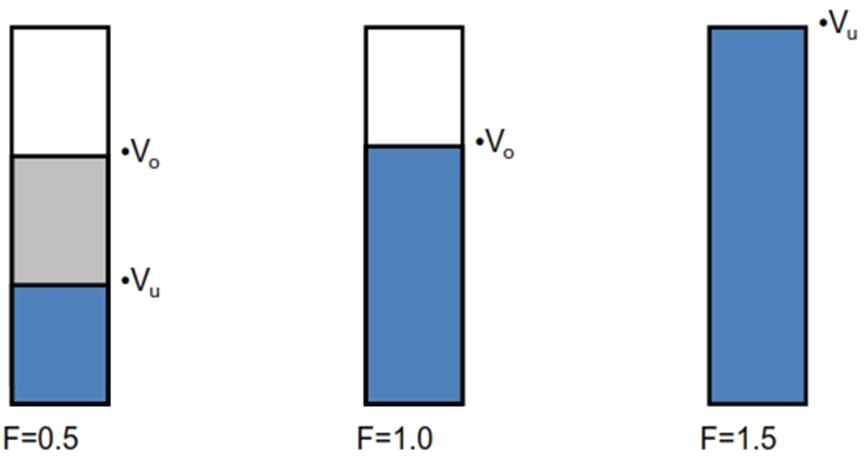
Sediment Volume
F= {volume of sediment Vu}/{original volume Vo}
1. Sedimentation volume
F = Vu/Vo
Vu – Volume of sediment
VO – Total volume of suspension
• F = 1 is ideal and acceptable
2. Degree of flocculation
β = F/Fα
F = sedimentation volume for flocculated system
Fα = sedimentation volume for deflocculated system
Eg: if β = 3, the sedimentation volume in the flocculated suspenion is increased by 3 times the volume of sediment in the flocculated state
Interfacial Properties of Solids
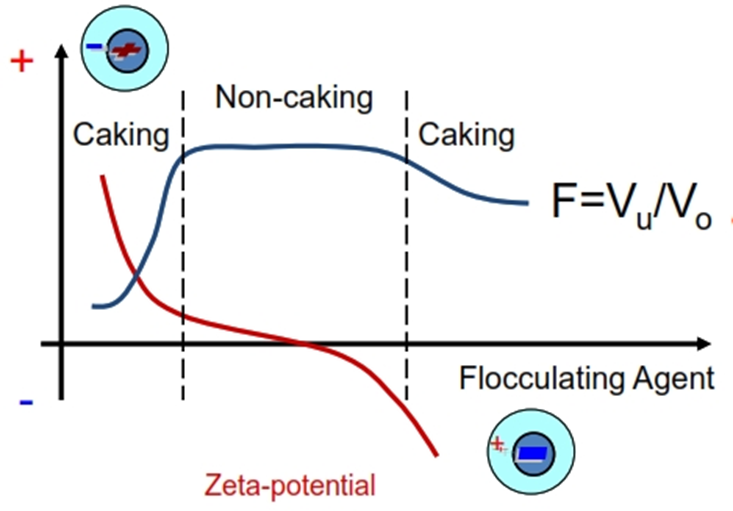
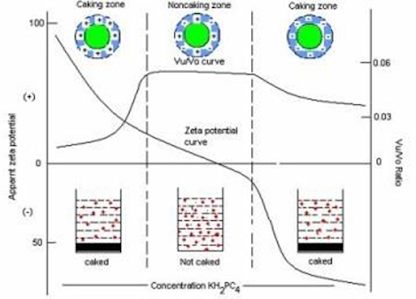
• Flocculation or caking
– determined by forces of attraction (van der Waals) versus forces of repulsion (electrostatic)
• Deflocculated
– Repulsion> attraction
– affected by [electrolytes]
• Flocculated
– Attraction > repulsion
Particle-Particle Interaction
Deflocculated Suspension
• Particles carry a definite charge
• Repulsion energy is high, that creates a high potential barrier which prevents aggregation of particles
• Particles are at Primary minimum
• During storage particles exhibit sedimentation and forms close packing
• Small particles fill in the void space between the larger particles
• As sedimentation continues lower portion of sediment gets pressed by weight of sediment above
• This force is sufficient to overcome high energy barrier
• Once this energy barrier is crossed, the particles comes in close contact with each other and establish stronger attraction forces
• Particles settle slowly, sediment is compressed over time to form a cake (aggregation)
• Difficult to re-suspend caked sediment by agitation
• Forms a turbid supernatant
Flocculated Suspension
• Particles are at Secondary minimum
• Weakly bonded to form fluffy conglomerates
• Loose structures with high energy barrier
• 3-D structure (gel-like)
• Settle rapidly but will not form a cake – resist close-packing, easily re-suspended
• Particle-particle interaction is influenced by ionic strength, pH, size and valency of added electrolytes
Electrical Properties
• Particles may become charged by
– Adsorption of ionic species present in solution or preferential adsorption of OH-
– Ionization of -COOH or -NH2 group
Electric Double Layer
Nernst potential
• Potential difference between the actual solid surface and the electroneutral bulk
Zeta potential
• Potential difference between the tightly bound layer and the bulk
• Governs electrostatic force of repulsion between solid particles
DLVO Theory: Optimal Distance
DERJAGUIN, LANDAU, VERWEY, OVERBEEK
Controlled Flocculation
• Flocculating agent changes zeta- potential of the particles (it can be electrolyte, charged surfactant or charged polymer adsorbing on a surface).
• If the absolute value of the zeta- potential is too high the system deflocculates because of increased repulsion and the dispersion cakes.
• Electrolytes
– Most widely used
– reduce zeta potential
• decrease force of repulsion
– change pH
– Bridge formation
• Alcohol
– Reduction in zeta potential
• Surfactants
– form adsorbed monolayers on particle surface
– Efficacy is dependent on charge, concentration
• Polymers
– Bdsorb to particle surface
– Bridging
– Viscosity, thixotropy
– Protective colloid action
– Most effective
Structured Vehicles
• Pseudoplastic or plastic dispersion medium
• Examples
– Methylcellulose, bentonite
• Negatively charged
• Increase viscosity
Combined Approach
• Possibility of incompatibilities of suspending agent and flocculating agent
– Structured vehicles have negative charge
– Incompatible if particle carries a negative charge
Rheology of Suspensions
• Flocculated particles in concentrated suspensions
– Exhibit pseudoplastic or plastic flow
• System resists flow until a yield stress is reached
• Below s substance is a solid
• Deflocculated systems exhibit Newtonian behavior
•Viscosity
– Increasing viscosity decreases rate of drug absorption
– Extent of absorption is unaffected, but may reduce effectiveness of drugs with a low therapeutic window
Formulation of Suspensions
• Two common approaches:
1. Use of a structured vehicle
– caking still a problem
2. Flocculation
– No cake formation
• Less common approach is to combine above
Preparation of Suspensions
• Reduce drug powder to desired size
• Add drug and wetting agent to solution
• Prepare solution of suspending agent
• Add other ingredients
– Electrolytes, color, flavor
• Homogenize medium
• Package
Evaluating Suspensions
• Two parameters
– Sedimentation volume, F = Vu/Vo
• Vu = final sediment volume
• Vo = initial dispersion volume
• want F =1
– Degree of flocculation, β= Vu/Vu
• Vu final sediment volume of deflocculated suspension
– Redispersibility – by rotation
– Micromertic property – Particle size
– Electrokinetic property – Zeta potential measurement
– Rheology – viscosity by Brookfield Viscometer
– Centrifugation – at 5000rpm for 5 minutes
– Freeze thaw cycling – Successive heating and cooling cycles (0 to 40 DegreeC)
– Accelerated stability testing – ICH guidelines – 45±2 Degree C with 75±5%RH – 6 months
Packaging and Storage of Suspensions
• All suspensions should be packaged in wide mouth containers having adequate airspace above the liquid to permit adequate shaking and ease of pouring.
• Most suspensions should be stored in tight containers protected from freezing, excessive heat, and light.
• It is important that suspensions be shaken before each use to ensure a uniform distribution of solid in the vehicle.
Summary
• Suspension: Solid drug in liquid vehicle
• Emulsion: Liquid drug in liquid vehicle
• Reasons for use of suspension include Drug is insoluble, more stable in suspension or emulsion, need to control the rate of release of the drug and has bad taste
• A properly prepared pharmaceutical suspension should settle slowly and should be readily redispersed upon gentle shaking of the container.
• The particle size of the suspension should remain fairly constant throughout long periods of undisturbed standing.
• The suspension should pour readily and evenly from its container.
• Stokes’ Equation
dx/dt=d2(ρi–ρe)g/18h
• Factors can be adjusted to enhance the physical stability of a suspension, includes the diameter of the particles, the density and viscosity of the medium.
• In most good pharmaceutical suspensions, the particle diameter is 1 to 50 mm.
• Particle size reduction is generally accomplished by dry milling prior to incorporation of the dispersed phase into the dispersion medium.
• The suspension shall form loose networks of flocks that settle rapidly, do not form cakes and are easy to resuspend.
• Brownian motion is based on size of particles ( up to 5micron size) and it depends on viscosity and density of medium
• Particles become charged by adsorption of ionic species present in solution or preferential adsorption of OH- and ionization of -COOH or –NH group
• Nernst potential is the potential difference between the actual solid surface and the electroneutral bulk
• Zeta potential is the potential difference between the tightly bound layer and the bulk and it governs electrostatic force of repulsion between solid particles
• Suspensions should be packaged in wide mouth containers having adequate airspace above the liquid to permit adequate shaking and ease of pouring.
• Most suspensions should be stored in tight containers protected from freezing, excessive heat, and light.
Also, Visit: Biotechnology Notes