Isolation and Fusion of Protoplast

Objective
At the end of the lecture, the student will be able to:
• Discuss the methods of protoplast isolation, culture and fusion
Isolation and Fusion of Protoplast
Isolation of Protoplast
General Introduction:
• Protoplast is a cell without cell wall/rigid cell wall/cells from which cell wall has been removed
• Isolation and fusion of protoplast is one of the significant development in tissue culture field
• Recent development is regeneration of whole plant from protoplasts
Mechanical method of Isolation of Protoplast:
• Crude method
• Cells are placed in a plasmolyticum solution
• Cells are then cut with a fine knife, there by protoplasts are released
• Used only for cells with large vacuoles like onion bulb, radish etc
• Not suitable for cells with small vacuoles
• Poor yield of protoplasts
Enzymatic method of Isolation of Protoplast:
• Widely used method
• Used for variety of tissues and organs including leaves, roots, stems, petioles, fruits, shoots etc
• Mesophyll tissue – most suitable source
• High yield of protoplast
• Easy to perform
• More protoplast viability
i. Sterilization of leaves
• Fully expanded leaves
• 70% alcohol for 1 min
• Sodium hypochlorite, 10-30 min
• Wash with sterile distilled water thrice
ii. Peeling of epidermis
• It is done using forceps
• Lower epidermis is peeled off
• Leaves are cut into small segments
• Mesophyll protoplasts are released from segments
• Epidermal protoplasts from epidermis
iii. Enzymatic treatment
• Cell wall degrading enzymes are used
a. Direct (one-step method)
• Leaf segments are incubated with enzyme mixture A (0.5% macerozyme + 2% cellulase in 13% mannitol, pH 5.4), 15-18 hrs
• Filtered using nylon mesh in order to remove cell debris /cell organelles
• Centrifuged at 100 g for 1 min, Protoplast forms a pellet and settled
• Washed with 13% mannitol and 20% sucrose
• Centrifuged again at 200g for 5 min
• Protoplasts float and can be pipeted out
b. Sequential method (Two step method)
• Leaf segments are infiltrated with enzyme mixture A (0.5% macerozyme + 0.3% potassium dextran, pH 5.8), 15 min
• Replaced by fresh enzyme mixture and heated on water bath for 1 hr
• Filtered through nylon mesh and centrifuged at 100g for 1 min, protoplasts form pellets
• Cells are mixed with enzyme mixture B (2% cellulase in 13% mannitol) incubated at 60 0C for 90 min
• Filtered and centrifuged at 100 g for 1 min, repeat the same procedure as in direct method
iv. Purification
a. Sedimentation and washing
• Protoplasts are subjected to slow centrifugation at 50 g for 5 min
• Allows protoplast to sediment and then washed
b. Flotation method
• Gradients are used
• Subjected to centrifugation, protoplasts float
• A solution of mannitol, sorbitol and sucrose (0.3- 0.6 M) used as gradients
Protoplast Culture
• Isolated protoplasts can be cultured in a suitable nutrient medium to regenerate cell wall and callus
• Optimal culture conditions are
– Optimal auxin to cytokinin ratio
– Maintenance of osmotic pressure
– Temp – 20-28 0C, pH – 5.5-5.9
– NAA and BAP
• Cell wall regenerated within 5-7 days followed by microscopic colonies
• Formation of cell wall – stain with calciflour white stain- green fluorescence
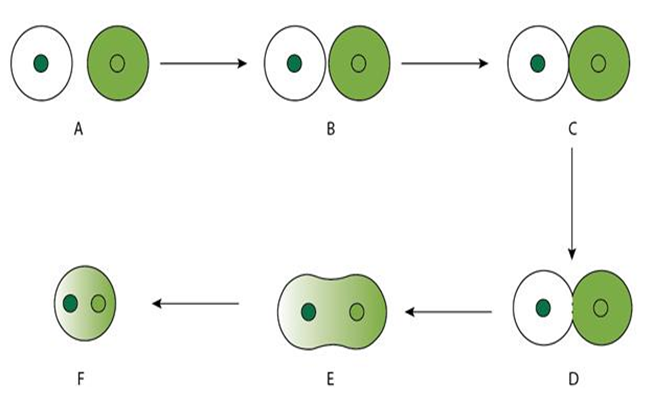
Spontaneous fusion:
• Occurs naturally
• During isolation, protoplasts lying in close proximity undergo fusion themselves à Homokaryons or homokaryocytes
• Mechanically can also be induced by bringing protoplasts close by using micro manipulator
Induced fusion:
• Induced artificially – fusogens
• Induced by using certain chemicals like
– Sodium nitrate
– Calcium ions
– PEG
a. Treatment with sodium nitrate
• Isolated protoplasts are suspended in 10% NaNO3
• Incubated at 35 0C for 30 min
• To obtain higher frequency, protoplasts can be centrifuged and subjected to 2 more cycles
b. Treatment with Ca++
• Isolated protoplasts are suspended in 0.1 M Calcium chloride in 0.4 M mannitol, pH 10.5
• Centrifuged at 50 g for 30 min, incubated on water bath for 30 min
c. Treatment with PEG
I Method:
• When protoplasts are available in sufficient quantity, 1 ml of medium containing protoplast is mixed with 1 ml of 55% PEG, incubated at 35 0C for 30 min
II Method:
• When available in small quantity, 4-8 µl is taken in petridish and allowed to settle and added 2-3 µl of PEG, incubated at 35 0C for 30 min
Electro fusion:
• Mild electrical current is used
• Two glass micro electrode are placed in contact with protoplasts
• Apply of electric field of low strength leads to pearl chain arrangement of protoplasts
• Subsequent application of high strength leads to fusion
Summary
• Protoplast – Cell without a cell wall
• Isolation of protoplast – Mechanical method and enzymatic method
• Enzymatic method – Direct method and sequential method
• Cell wall degrading enzymes – Cellulase, pectinase, macerozyme etc
• Purification by washing and sedimentation method and flotation method
• Protoplast culture – regeneration of cell wall on a suitable media
• Protoplast fusion – Spontaneous and induced fusion