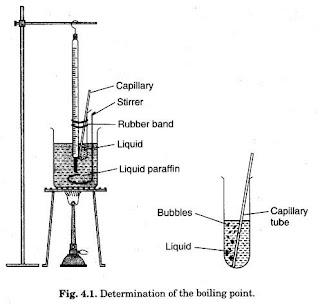
Determination of Boiling Point

Determination of Boiling Point
The boiling point is a fundamental concept in chemistry and physics, playing a crucial role in various scientific disciplines and everyday life. In this article, we will delve into the concept of boiling point, its determination, and its significance in a wide range of applications.
Introduction
Boiling point is the temperature at which a substance changes from a liquid to a gas, typically under standard atmospheric pressure. It’s a property that can vary widely among different substances and is a key characteristic used for their identification and analysis.
What is Boiling Point?
The boiling point is the temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure. At this temperature, the liquid molecules have enough energy to break their bonds and become a gas.
Factors Affecting Boiling Point
The boiling point of a substance is influenced by several factors, including intermolecular forces, molecular weight, and the presence of impurities.
Importance of Boiling Point
The boiling point is a vital property in chemistry. It helps in the separation and purification of substances, determining the purity of compounds, and understanding their behavior under different conditions.
The boiling point of a liquid is the corrected temperature at which the vapor pressure of the liquid reaches 101.3 kPa.
Method for Determination of Boiling Point:
Simple Distillation
This method is used to separate components from a mixture based on their differing boiling points. It is a straightforward technique that finds applications in laboratories and industrial processes.
Place in the flask 20 ml of the liquid being examined, add a few pieces of porous material, and heat rapidly to boiling.
Record the temperature at which liquid begins to run from the side arm into the condenser.
Correct the temperature reading for the effect of barometric pressure using the following expression:
t1 = t2 + K (101.3 – b)
Where,
t1 = the correct temperature
t2 = the measured temperature
B = the barometric pressure in kPa at the time of the determination
K = the correction factor
Fractional Distillation
Fractional distillation is employed when the components of a mixture have closer boiling points. It provides a higher degree of separation and is commonly used in the petrochemical industry.
Refractive Index Method
This method measures the refractive index of a liquid as it is heated. The change in refractive index with temperature is used to determine the boiling point.
Practical Applications of Boiling Point
Pharmaceutical Industry
Pharmaceutical companies use the boiling point to separate and purify compounds, ensuring the safety and efficacy of medications.
Food Processing
In the food industry, the boiling point is crucial for cooking, preserving, and processing various food products.
Conclusion
In conclusion, the determination of boiling point is a fundamental aspect of chemistry, with wide-ranging applications across various industries. Its understanding is critical for the identification, separation, and purification of substances.
Frequently Asked Questions (FAQs)
Q1: Can the boiling point of a substance change under different pressures?
Yes, the boiling point of a substance can vary with changes in pressure. Higher pressures generally lead to higher boiling points, while lower pressures result in lower boiling points.
Q2: How is the boiling point used in the production of alcoholic beverages?
The boiling point is crucial in distillation processes used to create alcoholic beverages, separating alcohol from water and other components.
Q3: Are there exceptions to the rule that substances boil at a specific temperature?
Yes, some substances can exhibit multiple boiling points or broad boiling point ranges due to the presence of impurities or chemical reactions.
Q4: What is the significance of the boiling point in the oil and gas industry?
In the oil and gas sector, the boiling point is used in fractional distillation to separate crude oil into its various components, including gasoline, diesel, and kerosene.
Q5: How does the boiling point relate to the concept of vapor pressure?
The boiling point is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure, signifying the transition from a liquid to a gas phase.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf