ACTIVE PHAMACEUTICAL
INGREDIENT SPECIFICATION
ACECLOFENAC
IP
Synonyms
: [[[2-[(2,6-Dichlorophenyl)amino]phenyl]acetyl]oxy]acetic
acid.
SAMPLING AND HANDLING
STORAGE REQUIREMENT
Store protected
from light.
SAMPLING
Carry out
the sampling as per SOP
QUANTITY OF COMPOSITE SAMPLE FOR ANALYSIS
15 g
QUANTITY OF RESERVE SAMPLE
2 x 15 g
RETEST PERIOD
One year
HAZARDS AND PRECAUTIONS, IF ANY
Use hand
gloves and nose mask while sampling.
DISPOSITION OF ANALYTICAL SAMPLE
Carry out
disposition of analytical sample as per SOP
STANDARDS
Molecular Formula: C16H13Cl2
NO4 Molecular weight : 354.20
DESCRIPTION: A White or almost white,
crystalline powder.
SOLUBILITY: Freely soluble in acetone,
Soluble in alcohol, chloroform; practically insoluble in water.
IDENTIFICATION:
Test A may
be omitted if tests B and C are carried out. Tests B and C may be omitted if
test A is carried out.
A] The infrared absorption spectrum of
the residue is concordant with the reference spectrum of aceclofenac or with
the spectrum obtained from aceclofenac RS.
B] When
examined in the range of 220 nm to 370 nm, the 0.002% w/v solution in methanol
shows an absorption maximum at 275 nm.
C] A blue colour
develops and a precipitate is formed.
RELATED SUBSTANCES: In the chromatogram
obtained with test solution, the area of any secondary peak it not more than
0.5 times the area of peak in the chromatogram obtained with reference solution
(b) (0.5%) and the sum of areas of all secondary peaks is not more than the
area of the peak in the chromatogram obtained with the reference solution (b)
(2.0%).
HEAVY METALS: Not more than 10 ppm.
SULPHATED ASH: Not more than 0.1%
LOSS ON DRYING: Not more than 0.5% w/w
ASSAY: Aceclofenac contains: Not less
than 99.0% and not more than 101.0%, calculated with reference to the dried
substances.
Note: * Represents test to be carried out
while retesting.
TESTS AND METHODS
DESCRIPTION: Examine the composite
samples and note the observations.
SOLUBILITY:
Freely soluble
in acetone (1 g in 10
ml)
Soluble in
methanol (1 g in 30 ml)
Practically
insoluble in water (0.01 gm in 100 ml
and over)
IDENTIFICATION:
Test A may
be omitted if tests B and C are carried out. Tests B and C may be omitted if
test A is carried out.
A) The infrared absorption spectrum of the residue is
concordant with the reference spectrum of aceclofenac or with the spectrum obtained from
aceclofenac RS.
B) When examined in the range of 220 nm to 370 nm, the
0.002% w/v solution in methanol shows an absorption maximum at 275 nm.
C) Dissolve about 10 mg in 10 ml of alcohol R. To 1
ml of the solution, add 0.2 ml of a mixture, prepared immediately before use,
of equal volumes of a 6 g/l solution of potassium ferricyanide R and a 9
g/l solution of ferric chloride R. Allow to stand protected from light
for 5 min. Add 3 ml of a 10.0 g/l solution of hydrochloric acid R. Allow
to stand protected from light for 15 min. A blue colour develops and a
precipitate is formed.
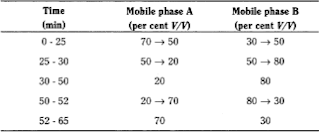
RELATED SUBSTANCES: By Liquid
chromatography
Prepare
the solutions immediately before use.
Test
solution Dissolve 50.0 mg of the substance to be examined in a mixture of
30 volumes of mobile phase A and 70 volumes of mobile phase B and dilute to
25.0 ml with the same mixture of solvents.
Reference
solution (a) Dissolve 21.6 mg of diclofenac sodium CRS in a mixture
of 30 volumes of mobile phase A and 70
volumes of mobile phase B and dilute to 50.0
ml with the same mixture of solvents.
RELATED SUBSTANCES CONTD.
Reference
solution (b) Dilute 2.0 ml of the test solution to 10.0 ml with a mixture
of 30 volumes of mobile phase A and 70
volumes of mobile phase B.
Reference
solution (c) Mix 1.0 ml of reference solution (a) and 1.0 ml of
reference solution (b) and dilute to
100.0 ml with a mixture of 30 volumes of mobile phase A and 70 volumes of mobile phase B.
Reference
solution (d) Dissolve 4.0 mg of aceclofenac impurity F CRS, 2.0 mg
of aceclofenac impurity H CRS and
2.0 mg of diclofenac impurity A CRS (aceclofenac impurity I) in a mixture of 30 volumes of
mobile phase A and 70 volumes of mobile
phase B and dilute to 10.0 ml with the same mixture of solvents.
Reference
solution (e) Mix 1.0 ml of reference solution (b) and 1.0 ml of reference solution (d) and dilute to 100.0 ml with a
mixture of 30 volumes of mobile phase A
and 70 volumes of mobile phase B.
Column:
—size: l = 0.25 m, Ø = 4.6 mm,
—stationary phase: spherical end-capped
octadecylsilyl silica gel for chromatography R (5 µm) with a pore size of
10 nm and a carbon loading of 19 per cent,
—temperature: 40 °C.
Mobile phase:
—Mobile phase A: 1.12 g/l solution of phosphoric
acid R adjusted to pH 7.0 using a 42 g/l solution of sodium hydroxide R,
—Mobile phase B: water R, acetonitrile
R (1:9 V/V),
Flow
rate 1.0 ml/min.
Detection Spectrophotometer
at 275 nm.
Injection 10
µl; inject the test solution and reference solutions (c) and (e).
Relative
retention with reference to aceclofenac (retention time = about 14
min): impurity A = about 0.8; impurity G
= about 1.3; impurity H = about 1.5; impurity I = about 2.3; impurity D = about
2.6; impurity B = about 2.7; impurity E = about 2.8; impurity C = about 3.0;
impurity F = about 3.2.
System suitability Reference solution
(c):
—Resolution: minimum 5.0 between the
peaks due to aceclofenac and to impurity A.
Limits:
—impurity A: not more than the area of
the corresponding peak in the chromatogram obtained with reference solution (c)
(0.2 per cent),
—impurities B, C, D, E, G: for each
impurity, not more than the area of the peak due to aceclofenac in the
chromatogram obtained with reference solution (e) (0.2 per cent),
—impurity F: not more than the area of
the corresponding peak in the chromatogram obtained with reference solution (e)
(0.2 per cent),
—impurity
H: not more than the area of the corresponding peak in the chromatogram
obtained with reference solution (e) (0.1 per cent),
—impurity I: not more than the area
of the corresponding peak in the chromatogram obtained with reference solution
(e) (0.1 per cent),
—any other impurity: not more than
half the area of the peak due to aceclofenac in the chromatogram obtained with
reference solution (e) (0.1 per cent),
—total: not more than 0.7 per cent,
—disregard limit: 0.1 times the area
of the peak due to aceclofenac in the chromatogram obtained with reference
solution (e) (0.02 per cent).
HEAVY METALS: (Refer GTP/23):
To 2.0 g in
a silica crucible, add 2 ml of sulphuric acid R to wet the substance. Heat
progressively to ignition and continue heating until an almost white or at most
a greyish residue is obtained. Carry out the ignition at a temperature not exceeding
800 °C. Allow to cool. Add 3 ml of hydrochloric acid R and 1 ml of nitric
acid R. Heat and evaporate slowly to dryness. Cool and add 1 ml of a 100
g/l solution of hydrochloric acid R
and 10.0 ml of distilled water R. Neutralise with a 1.0 g/l
solution of ammonia R using 0.1
ml of phenolphthalein solution R as indicator. Add 2.0 ml of a 60 g/l
solution of anhydrous acetic acid R and dilute to 20 ml with distilled
water R. 12 ml of the solution
complies with limit test A. Prepare the standard using lead standard
solution (1 ppm Pb) R.
SULPHATED ASH: (Refer GTP/09):
Determine on 1.0 g of the sample.
LOSS ON DRYING: (Refer GTP/07):
Determine on 1.0 g of the sample at 105 0 C.
ASSAY: Dissolve 0.300 g in 40 ml of methanol
R. Titrate with 0.1 M sodium hydroxide, determining the end-point
potentiometrically.
Each ml of 0.1
M sodium hydroxide is equivalent to 35.42 mg of C16H13Cl2NO4.
Calculation:
Corrected titre x 0.03542 x Molarity
factor of 0.1M sodium hydroxide x 100 x 100
= ——————————————————————————————————-
Wt. of Spl (g) x (100 – % L.O.D)