Freeze
Dryer
It is also known as lyophilization i.e. system is made
solvent loving for removing the same.
Principle
• In freeze drying, water is removed from the frozen state
by Sublimation i.e. direct change of water from solid into vapour without
conversion to liquid phase.
• Solid-liquid- vapour equilibrium phase diagram of water is
useful to decide the experimental conditions. The drying is achieved by
subjecting material to temperature & pressure below the triple point.
• Under this conditions, any heat transferred is used as
latent heat & ice sublimes directly into vapour state.
• The water vapour is removed from the system by
condensation in a cold trap maintained at a temperature lower than frozen
material.
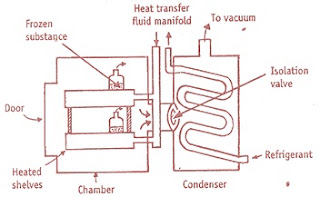
Construction
Freeze dryer consist of
• Drying chamber in which trays are locked
• Heat supply in the form of radiation source, heating coils
• Vapour condensing or adsorption system
• Vacuum pump or steam ejector or both
Working
The working of freeze dryer consist of following steps;
1. Preparation &
pretreatment
The volume of solution introduced into the container is
limited by its capacity. Therefore pretreatment is essential. The solutions are
preconcentrated under the normal vacuum tray drying. This reduces the actual
drying by 8 to 10 times.
2. Prefreezing to
solidify water
Vials, ampoules or bottles in which the aqueous solution is
packed are frozen in cold shelves (- 50ᵒC).
The normal cooling rate is about 1 to 3 Kelvin/ minute so
that large ice crystals with relatively large holes are formed on sublimation
of ice. This is also responsible for giving a porous product.
3. Primary Drying
• It means sublimation of ice under vacuum. The temperature
& pressure should be below the triple point of water i.e. 0.0098ᵒC &
4.58 mmHg for sublimation, when water is alone present
• When a Solution of a solid is dried, the depression of
freezing point of water occurs. Hence, it is essential that the temperature be
brought below the eutectic point. The pressure & temp. at which the frozen
solid vaporizes without conversion to liquid is referred to as the eutectic
point. Depending on the drug substances dissolved in water, the eutectic point
is determined. The usual range is from -10ᵒC to -30ᵒC.
The conditions of 1 to 8 K below eutectic point is
sufficient.
• Vacuum is applied to the tune of about 3 mmHg on the
frozen sample and the temperature is linearly increased about 30 ᵒC in a span
of 2 hrs. Heat (About 2900 kilojoules/ Kg) is supplied which transfer as latent
heat & ice sublimes directly into vapour state. As the drying proceeds,
thickness of dried solids increases. Primary drying stage removes easily
removable water, about 98% to 99%.
4. Secondary Drying
It is removable of residual moisture under high Vacuum. The
temp. of solid is raised to as high as 50 to 60ᵒC but vacuum is lowered below
that is used in primary drying.
The rate of drying is very low & it takes about 10 to 20
hrs
5. Packing
After vacuum is replaced by inert gas, the bottles &
vials are closed
Advantages
1. Thermo labile substances can be dried
2. Denaturation does not occur
3. Migration of salts & other solutes does not take
place
4. Moisture level can be kept as low as possible
5. Product is porous & uniform
6. Sterility can be maintained
7. Material can be dried in its final container such as
single dose & multiple dose
Disadvantages
1. The product is prone to oxidation, due to high porosity
& large surface area. Therefore, the product should be packed in vacuum or
using inert gas.
2. Equipment & running cost is very high.
3. The period of drying is very high. Time cannot be
shortened.
4. It is difficult to adopt the method for solutions
containing non-aqueous solvents.
Uses
It is used for drying of number of product;
1. Blood plasma & its fractionated product
2. Bacterial & viral culture
3. Antibiotics & plant extracts
4. Steroids, vitamins & enzymes