Ocular drug delivery system
Intended
Learning Objectives
At the end of this session, students will be able to
• Discuss about
human eye
• Enlist ocular
dosage forms
• Analyse the pros
and cons of topical ocular administration of medicaments
• Explain the routes
of drug absorption after topical administration
• Discuss the
conventional topical ocular dosage forms
• Classification
ocular inserts
• Explain the design
and application of Ocusert
• Discuss erodible
ocular inserts
• Discuss Contact
lens as drug delivery device to the eye
• Enlist the QC
tests for ophthalmic dosage forms
• Briefly explain
packaging of eye drops
Ocular Drug
Delivery System
• Eye is a complex organ with unique anatomy and physiology
• The anatomy of the eye can be studied by dividing it into
anterior and posterior segments
• Anterior segment of the eye occupies approximately
one-third
• Remaining portion is occupied by the posterior segment
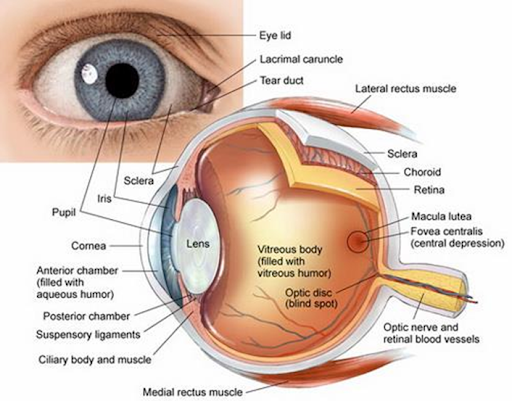
Anatomy and
Physiology of Human Eye
Anterior portion –
Cornea, Conjunctiva, Aqueous humor, Iris, Ciliary body, Lens
Posterior Portion –
Sclera, Choroid, Retina, Vitreous body
Cornea
• Devoid of blood vessels
• Derives nourishment form tear fluid and aqueous humor
• 12mm in diameter, 520µm in thickness
Conjunctiva
• Vascularized mucous membrane
• Lines the inner surface of the eyelids
• Generates mucous – Facilitates lubrication, helps with
tear film adhesion
Sclera
• Whitish outermost layer
• Composed of collagen bundles, mucopolysaccharides and
elastic fibres
• 10 times more permeable than cornea and half as permeable
as the conjunctiva
Fate of
Drugs Delivered by Ocular Route
Common
Conditions Affecting the Eye
Anterior segment –
Glaucoma, Allergic conjunctivitis, Anterior uveitis, Cataract
Posterior segment-
Age-related macular degeneration (AMD), Diabetic retinopathy
Ocular drug
delivery routes
Barriers
for Ocular Drug Absorption
Depending on the
route of administration
1. Topical
Precorneal factors
Solution
drainage
Blinking
Tear film
Tear turn over
Induced
lacrimation
Physical barriers
Cornea
Sclera
Conjuctiva
2. Oral
3. Periocular and
intravitreal
4. Parentetal
Blood aqueous
barrier
Blood retinal
barrier
Barriers
for Ocular Drug Absorption – Topical Route
➢ Mostly in the form of eye
drops
➢ Employed to treat anterior
segment diseases
➢ Site of action is usually
different layers of the cornea, conjunctiva, sclera, iris and ciliary body
(anterior uvea)
➢ Precorneal factors
– Solution drainage, blinking, tear film, tear turn over,
and induced lacrimation
– Human tear volume is estimated to be 7 μl
– Mucin present in the tear film plays a protective role by
forming a hydrophilic layer that moves over the glycocalyx of the ocular
surface and clears debris and pathogens
– Contact time with the absorptive membranes is lower
– Less than 5% of the applied dose reaches the intraocular
tissues
Mechanical barriers
for topical drug absorption
Cornea
• Limits the entry of exogenous substances into the eye and
protects the ocular tissues
• Divided into the epithelium, stroma, and endothelium
• The corneal epithelium is lipoidal in nature
• Offers resistance for permeation of topically administered
hydrophilic drugs
Corneal epithelium…
• Corneal epithelial cells are joined to one another by
desmosomes
• Tight junctional complexes retards paracellular drug
permeation from the tear film into intercellular spaces of the epithelium as
well as inner layers of the cornea
Layers of the Cornea
Stroma
➢ Comprises 90% of the corneal
thickness
➢ Highly hydrated structure
➢ Barrier to permeation of
lipophilic drug molecules
Endothelium
➢ Endothelial junctions are
leaky – facilitate the passage of macromolecules between the aqueous humor and
stroma
➢ Drugs should have an
amphipathic nature in order to permeate through these layers
Conjunctival drug
absorption
➢ Considered to be
non-productive
➢ Conjunctival blood capillaries
and lymphatics, which can cause significant drug loss into the systemic
circulation
➢ Conjunctival epithelial tight
junctions further retard passive movement of hydrophilic molecules
Barriers
for Ocular Drug Absorption – Topical Absorption
Sclera
➢ Consists of collagen fibers
and proteoglycans embedded in an extracellular matrix
➢ Permeability – comparable to
that of the corneal stroma
➢ Positively charged molecules
exhibit poor permeability presumably due to their binding to the negatively
charged proteoglycan matrix
➢ Permeability of drug molecules
across the sclera is inversely proportional to the molecular radius
Barriers
for Ocular Drug Absorption – Parenteral Route
• Anterior segment: blood–aqueous barrier
• Posterior segment: blood–retinal barrier
Blood–aqueous barrier
• Tight junctional complexes and prevent the entry of
solutes into the intraocular environment such as the aqueous humor
Blood–retinal barrier
• Restricts the entry of the therapeutic agents from blood
into the posterior segment.
• Regulates drug permeation from blood to the retina
Barriers
for Ocular Drug Absorption – Oral Route
• Limited accessibility to many of the targeted ocular
tissues limits the utility of oral administration
• Necessitates high dosage to observe significant
therapeutic efficacy
• Can result in systemic side effects
Barriers
for Ocular Drug Absorption – Periocular and Intravitreal Administration
• To overcome the inefficiency of topical and systemic
dosing to deliver therapeutic drug concentrations to the posterior segment
• The periocular route includes
– subconjunctival, subtenon, retrobulbar, and peribulbar
administration
• Comparatively less invasive than intravitreal route
Ocular
Dosage forms
• They are specialized dosage forms designed to be instilled
onto the external surface of the eye (topical), administered inside
(intraocular) or adjacent (periocular) to the eye or used in conjunction with
an ophthalmic device
• The most commonly employed ophthalmic dosage forms are solutions,
suspensions, and ointments
• The newest dosage forms for ophthalmic drug delivery are:
gels, gel-forming solutions, ocular inserts, intravitreal injections and
implants
Ocular Drug
Delivery Systems
1. Liquids
Solutions
Suspensions
Powders for
reconstitution
Sol to gel
systems
2. Semisolids
Ointments
Gels
3. Solid
Ocular inserts
Contact lens
4. Intraocular dosage
form
Injections
Irrigating
solutions
Implants
Topical
Application
• Applying the drug
product to the ocular surface, where it mixes with the lacrimal fluid
• Used to treat
anterior segment diseases
Ocular surface
• Dry eye disease or infections – Needs retention of drug in
tear film
Cornea and
conjunctiva
• Infection, inflammation, or neovascularization – Absorbed
by the cornea or conjunctiva
Tissues surrounding
the anterior chamber
• Elevated intraocular pressure, inflammation, or infection
– Permeate across the cornea and/or conjunctiva
Topical
Application Advantages
• The administration
of the dosage form locally to the eye may be easily performed by the patient
• The application of the therapeutic agents directly to the
site of action ensures that the therapeutic agent is available at higher
concentrations than may be achieved following oral administration
• They have quick absorption and less visual and systemic
side effects
Topical
Application Disadvantages
• The very short time the solution stays at the eye surface
• Poor bioavailability
• The instability of the dissolved drug
• The necessity of using preservative
Absorption
of topically applied drugs
• Corneal route
– Drug Instillation
– Dilution in tear fluid
– Diffusion from mucin layer
– Corneal penetration
– Diffusion into aqueous humor
• Non corneal route
– Conjuctival route
– Scleral route
Non corneal
route
Through the
conjunctiva and sclera → Iris and ciliary body
This route is important
for the absorption of hydrophilic small molecules, and a viable option for
large molecules, because the intercellular spaces in the conjunctival
epithelium are wider than in the cornea, being more permeable to larger
molecules
In the conjunctiva, compounds with molecular weights up to 5
kDa are able to permeate, whereas the sclera allows passage of macromolecules
(e.g., molecular weight of 100 kDa)
Corneal
Route
• The bioavailability of topically applied ocular drugs in
the aqueous humor is usually in the range of 0.001–0.05 (i.e. 0.1–5%)
Reasons
• Short retention of eye drops on the ocular surface
• Flow from the ocular surface to the nasal cavity
• Drug absorption across the conjunctiva and into the blood
stream (Example, 50% of instilled pilocarpine is absorbed from the lacrimal
fluid directly into the blood circulation)
• The intercellular tight junctions on the surface of the
corneal epithelium limit absorption of small molecules and block the permeation
of macromolecules, such as proteins
Characteristics
Required to Optimize Ocular Drug Delivery System
• Good corneal penetration
• Prolong contact time with corneal tissue
• Simplicity of instillation for the patient
• Non irritative and comfortable form (viscous solution
should not provoke lachrymal secretion and reflex blinking)
• Appropriate rheological properties concentrations of the viscous
system
Ocular
Dosage forms
Conventional
topical ocular dosage forms
• Eye drops/
solutions
• Suspensions
• Emulsions
• Ointments
Eye Drops/
Solutions
❖ The administration of
these to the
eye is usually
performed using a dropper (or a container with a dropper nozzle) or a
tube with a nozzle
Disadvantages –
Explained
• Retention of the
drug at the site of action is relatively Poor 7 µl for the blinking eye, 30 µl
for the non-blinking eye
➢ The typical
volume of two
drops of a
solution formulation is approximately 100 µl
and therefore the majority of the applied dose is lost either through spillage
on to the face or via the lacrimal duct
• To overcome these deficiencies in practice, the patient is
required to administer the ocular solution formulations (containing high
concentrations of therapeutic agent) frequently, which is inconvenient and may lead
to patient non-compliance
• Ocular
formulations are sterile and therefore specialised facilities are required for
the manufacture of these dosage forms
• Local side-effects may be experienced to ocular dosage
forms (to either the high concentration of therapeutic agent (5% w/w) or excipients
used in the formulation). Typically pain and irritation are the major
side-effects encountered by patients
Sawtooth Pattern of
Therapy Following Administration of Ophthalmic Drugs as Eye Drops
Methods to improve
ocular bioavailability with eye drops
1. Incorporating viscosity enhancers like HMC, HEC, sod CMC,
HPMC
Reduces solution
drainage and increases the contact time
2. Using permeation enhancers like benzalkonium chloride,
cyclodextrins, sod EDTA in the formulation
Improves permeation
across the corneal barrier
Aqueous
ophthalmic solution
▪ Manufactured by dissolution of the active ingredients and
a portion of the excipients into all portion of water
▪ The sterilization of this solution done by heat or by
sterilizing filtration through sterile depth or membrane filter media into a
sterile receptacle
▪ This sterile solution is then mixed with the additional
required sterile components such as viscosity –imparting agents, preservatives
and so and the solution is brought to final volume with additional sterile
water
Suspension
⚫ If the drug is not
sufficiently soluble, it can be formulated as a suspension
⚫ A suspension may also be
desired to improve stability, Bioavailability, and efficacy
⚫ The major topical ophthalmic
suspensions are the steroid anti-inflammatory agents
⚫ An ophthalmic suspension
should use the drug in a microfine form; usually 95% or more of the particles
have a diameter of 10µm or less
• Prednisolone acetate suspension
• Besifloxacin suspension
• Blephamidesuspension
• Fluorometholone
Advantages
• Patient compliance
• Best for drug with
slow dissolution
Disadvantages
• Drug properties
decide performance
• Loss of both
solution and suspended solid
Emulsion
Advantages
• Prolonged drug
release
Disadvantages
• Blurred vision
• Patient non
compliance
• Possible oil
entrapment
Packaging of eye
drops
• Ophthalmic liquids can be packaged in sterile glass
bottles with separate dropper or in plastic bottles with self-contained dropper
tips
Glass bottle packaging
• Dropper bottle for
eye drops are fitted with a cap, rubber teat and dropper as the closure
• The bottles are
used at a capacity of 10 ml or 20 ml
• Glass containers
are used in only a very few instances because of stability limitations
• Type 1
glass vials with
appropriate stoppers are
used for ophthalmic products
Plastic packaging
• Currently all most all commercially available ophthalmic
products are packaged in plastic containers
• Advantages of plastic containers are ease of use, little
breakage, less spillage. This led to universal acceptance of plastic containers.
• Plastic packaging components consists of bottle fitment
and closure
• It has multi-component single-drop dispenser. Eye drops
must be sterilezed after filling into
the containers and sealing, by autoclaving at a temperature of 90-100oC for 30
mins, or alternatively they may be pre sterilized and filled aseptically into
previously sterilised containers
• The containers are usually fitted with droppers attached to
the closures
Two types of dose
preparations in plastic packaging
• Single dose
preparations
• Multiple dose
preparations
Single dose
preparations
• The ideal type of packaging for eye drops is a disposable
one shot container which eliminates the need for any preservative and reduces
the risk of infecting the eye during applications almost to zero
• Single dose packs are available in which the solutions can
be sterilised by autoclaving in air ballasted autoclaves these solutions can
therefore be formulated without a preservative
• Single-use vials, when filled under sterile conditions,
have the additional advantage of enabling the product to be formulated without
preservatives
• Most products in
multi-use containers need preservatives to counteract microorganisms after each
use
Multiple dose
preparations
• Multiple dose preparations must contain an antimicrobial
preservative to prevent proliferation of contaminants during use and to support
the maintenance of sterility
• Examples of preservatives are phenyl mercuric nitrate or
acetate, chlorhexidine acetate or benzalkonium chloride
Eye
Ointment
• The ointment vehicles used in ophthalmology is mixture of
Mineral oil and petrolatum base
• The mineral oil
is used to
modify melting point
and modify consistency
• Petrolatum vehicle
used as a
ocular lubricate to
treat dry Eye syndromes
• They are mostly used as adjunct night time therapy, while
eye drops are administered during the day
• It is suitable for moisture sensitive drugs and has longer
contact time than drops
Manufacturing
Techniques
➢ The ointment base is
sterilized by heat and appropriately filtered while molten to remove foreign
particulate matter
➢ It is then placed into a
sterile steam jacket kettle to maintain the ointment in a molten state under
aseptic conditions, and the previously sterilized active ingredient(s) and
excipients are added aseptically
➢ The entire ointment may be
passed through a previously sterilized colloid mill for adequate dispersion of
the insoluble components. After the product is compounded in an aseptic manner,
it is filled into a previously sterilized container
Advantages
• Flexibility in
drug choice
• Improved drug
stability
Disadvantages
• Sticking of eye
lids
• Blurred vision
• Poor patient
compliance
• Drug choice
limited by partition coefficient
Packaging
Ophthalmic ointment are packaged in:
1. Small collapsible tin tube usually holding 3.5g of
product. The pure tin tube is compatible with a wide range of drugs in
petrolatum-based ointments
2. Aluminum tubes have been used because of their lower cost
and as an alternative
3. Plastic tubes made from flexible LDPE resins have also
been considered as an alternative material
• Filled tubes may
be tested for leakers
• The screw cap is
made of polyethylene or polypropylene
• The tube can be a
source of metal particles and must be cleaned carefully before sterilization
(by autoclaving or ethylene oxide)
Recent
Formulation Trends in Ocular Controlled Drug Delivery System
Ocular
Insert
Non
erodible inserts
• The Ocusert therapeutic system is a flat, flexible,
elliptical device designed to be placed in the inferior cul-de-sac between the
sclera and the eyelid and to release Pilocarpine continuously at a steady rate
for 7 days
Ocusert
The device consists of 3 layers…..
• Outer layer – ethylene vinyl acetate copolymer layer.
• Inner Core – Pilocarpine gelled with alginate main
polymer.
• A retaining ring – of EVA impregnated with titanium
dioxide
The ocuserts
available in two forms.
• Pilo – 20 (20
microgram / hour)
• Pilo – 40 (40
microgram / hour)
Use: Chronic
glaucoma
Advantages
• Reduced local side effects and toxicity.
• Around the clock control of IOP.
• Improved compliance.
Disadvantages
• Retention in the eye for the full 7 days.
• Periodical check of unit.
• Replacement of contaminated unit
• Expensive.
Erodible
Inserts
• The solid inserts absorb the aqueous tear fluid and
gradually erode or disintegrate
• The drug is slowly leached from the hydrophilic matrix
• They quickly lose their solid integrity and are squeezed
out of the eye with eye movement and blinking
• Do not have to be removed at the end of their use
Three types
• LACRISERTS
• SODI
• MINIDISC
1. LACRISERTS
• Sterile rod shaped device made up of hydroxy propyl
cellulose without any preservative
• For the treatment of dry eye syndromes
• It weighs 5 mg & measures 1.27 mm in diameter with a
length of 3.5 mm
• It is inserted
into the inferior fornix
2. SODI
– Soluble Ocular Drug Inserts
– Small oval wafer
– Sterile thin film of oval shape
– Weighs 15-16 mg
– Introduced into the inferior cul-de-sac.
Use – glaucoma
Advantage –
single application
3. MINIDISC
• Countered disc with
a convex front and a concave back surface
• Diameter – 4 to 5
mm
Composition
• Soluble copolymers consisting of actylamide, N-vinyl
pyrrolidone and ethyl acetate Iontophoresis
Contact
Lens
• Contact lenses are thin, and curved shape plastic disks which
are designed to cover the cornea
• After application, contact lens adheres to the film of
tears over the cornea due to the surface tension
• 1930 Polymethyl methacrylate (PMMA) was used as the first
successful contact lens (CL) material
• 1965 Use of soft contact lens (SCL) for ophthalmic drug
delivery (Sedlacek)
• 1960s Discovery of hydrogels (Witcherle and Lim)
• 1970,s benefits of CL for ocular drug delivery (Kaufman)
• Early Conventional Hydrogel (CH) CLs did not provide
adequate oxygen transmission to the cornea, resulting in hypoxia related
complications during overnight wear, limiting their long term therapeutic
potential
• 1990,s Highly oxygen permeable Silicone Hydrogel (SH) CLs
were introduced
Advantages of Contact
lens
• Located in the
immediate vicinity of the cornea
• Limited mixing in the tear film between the lens and the
cornea leads to a residence time of more than 30 minutes (Compared to 5min for eye drops)
• Increase in
bioavailability
Materials for Contact
Lens
• Hydrogels – good transmission of visible light, high
chemical and mechanical stability, reasonable cost and high oxygen
transmissibility
• Poly HEMA – water
content of about 38%
• Methacrylic acid (MAA) with HEMA, soft contact lenses (SCLs)
with different water contents, hardness, strength and oxygen permeabilities can
be created
Strategies/Techniques
for Contact Lens Based Drug Delivery System
Soaking Method
• Involves soaking the
preformed contact lenses in the drug solution, followed by drug uptake and
release in pre- and post-lens tear film
• Contact lenses have internal channels/cavity for receiving/accommodating
the drug molecules
• Drug loading depends on the water content, thickness of
lenses, the molecular weight of the drug, soaking time period and concentration
of drug in soaking solution
Limitations
• High molecular weight drugs or polymers like hyaluronic
acid, do not penetrate the aqueous channels of contact lenses and remain on the
surface only
• Contact lenses have low affinity for most of the
ophthalmic drugs like timolol maleate, olopatadine HCl, brimonidine tartrate,
etc.
• Effects of sterilization and packaging processes on the
stability of therapeutic contact lenses – may cause premature release of the
drug
Molecular Imprinting
(MI)
• Monomers are
polymerised in the presence of drug template followed by removal of the template
• Resulting in
formation of tailored active sites or imprinted pockets called macromolecular
memory sites
Limitation
• Highly cross-linked structure of hydrogel affects the
optical and physical performance of contact lens
• The drug-loading capacity is limited by the template
molecules and functional monomers, and the deformation (change in dimension) of
contact lenses after release of drug was also noted
• The fall in water content (decrease in swelling) leads to
an insufficient ion and oxygen permeability which limit the use of contact
lenses for extended wear
Quality
Control of Ophthalmic Products
• Universal tests
– Description
– Identification
– Assay
– Impurities
• IPQC & FPQC
– pH
– Isotonicity
– Viscosity
– Therapeutic efficacy
– Compatibility
– Clarity
– Particulate matter
– Insoluble particulate matter
– Particle size
– Uniformity of volume
– Uniformity of content
– Uniformity of weight
– Bacterial endotoxin
– Sterility testing
Summary
• Anatomy and
Physiology of Human Eye
Anterior portion –
Cornea, Conjunctiva, Aqueous humor, Iris, Ciliary body, Lens
Posterior Portion –
Sclera, Choroid, Retina, Vitreous body
• Common Conditions
Affecting the Eye
Anterior segment –
Glaucoma, Allergic conjunctivitis, Anterior uveitis, Cataract
Posterior segment-
Age-related macular degeneration (AMD), Diabetic retinopathy
• Conventional
topical ocular dosage forms
Eye drops/ solutions
Suspensions
Emulsions
Ointments
• Packaging of eye
drops
Ophthalmic liquids can be packaged in sterile glass bottles
with separate dropper or in plastic bottles with self-contained dropper tips
• Ocular inserts – Non-erodible and
erodible
• Non-erodible – Ocuserts and contact lens
• Erodible – Lacrisert, SODI, Minidisc
• Ocusert – Containing pilocarpine for
glaucoma treatment
• Lacrisert – Dry eye syndrome
• Contact lens
Contact lenses are thin, and curved shape plastic disks
which are designed to cover the cornea
After application, contact lens adheres to the film of tears
over the cornea due to the surface tension
• Advantages of
Contact lens
Located in the immediate vicinity of the cornea
Limited mixing in the tear film between the lens and the
cornea leads to a residence time of more than 30 minutes (Compared to 5min for
eye drops)
Increase in bioavailability
• Quality Control of
Ophthalmic Products
Universal tests
– Description
– Identification
– Assay
– Impurities
IPQC & FPQC
– pH
– Isotonicity
– Viscosity
– Therapeutic efficacy
– Compatibility
– Clarity
– Particulate matter
– Insoluble particulate matter
– Particle size
– Uniformity of volume
– Uniformity of content
– Uniformity of weight
– Bacterial endotoxin
– Sterility testing
For PDF Notes Click on Download Button