Polymers
Contents
• History
• Introduction
• Polymer synthesis
– Addition or free radical reaction
– Condensation reaction
• Classification of polymers
• Properties of polymers
• Ideal properties of polymers for pharmaceutical use
• Advantages of polymers
• Applications of polymers in pharmaceutical and biomedical
field
Learning
objectives
At the end of this chapter, student will be able to:
• Outline the historical development in polymer synthesis
• Define the terms, ‘polymer’, ‘monomer’, ‘degree of
polymerization’
• Describe polymer synthesis by free radical addition and
condensation reaction
• Classify polymers
• Describe the physical, mechanical and thermal properties
of polymers
• Enlist the ideal requirements of polymers for
pharmaceutical use
• Outline the advantages of polymers
• Discuss the applications of polymers in pharmaceutical and
biomedical field
History
• The first semisynthetic polymer ever made was guncotton
(cellulose nitrate) by Christian F. Schonbein in 1845
– Highly explosive
– Poor processability
– Poor solubility
• Celluloid (plasticized cellulose nitrate)
• Cellulose acetate (cellulose treated with acetic acid)
• Hydrolyzed cellulose acetate soluble in acetone
• In 1872, Bakelite, a strong and durable synthetic polymer based
on phenol and formaldehyde, was invented
• Other synthetic polymers invented later
– Polyethylene (1933)
– Poly (vinyl chloride) (1933)
– Polystyrene (1933)
– Polyamide (1935)
– Teflon (1938)
– Synthetic rubbers (1942)
Herman Staudinger, who received the Nobel Prize in Chemistry
in 1953, coined the term “macromolecule” in 1922 and used it in reference to
polymers.
What is the difference between ‘polymer’ and
‘macromolecule’?
Introduction
Single à
MONO
Many àPOLY
Polymers are high molecular weight compounds or molecules
composed of many repeating subunits called monomers, connected by covalent or
chemical bonds
• Polymerization –
Process of formation of macromolecules by linking of monomers together
• Degree of polymerization
(DP) –Average molecular weight of the polymer divided by the molecular
weight of the monomer
Polymer
properties
Determined by
• Length
• Molecular weight
• Backbone structure
• Side chain
Can polymers exist in gaseous state?
Modifications in properties of polymers
Changing molecular weight
Changing structure of monomer building blocks
Blending them with with other polymers
Polymer
Synthesis
Methods
– Addition polymerization
– Condensation polymerization
Addition
Polymerization/ Free-radical Polymerization
Monomer having a double bond
• The initiator is an unstable molecule that is cleaved into
two radical- carrying species under the action of heat, light, chemical, or
high-energy irradiation
• Each initiating radical has the ability to attack the
double bond of a monomer
• The π bond in a monomer generally requires low energy to
break; therefore, polymerization starts at this site by the addition of a free
radical on the monomer
• The radical is transferred to the monomer and a monomer
radical is produced. This step in polymerization is called initiation.
• The monomer radical is also able to attack another monomer
and then another monomer, and so on and so forth. This step is called
propagation by which a macroradical is formed.
• Macroradicals prepared in this way can undergo another
reaction with another macroradical or with another inert compound (e.g., an
impurity in the reaction) which terminates the macroradical.
Monomers such as acrylic acid, acrylamide, acrylic salts
(such as sodium acrylate), and acrylic esters (methyl acrylate) contain double
bonds and they can be polymerized via addition reactions.
Addition or
free-radical polymerization of styrene
Condensation
polymerization / step polymerization
• If a monomer
does not contain
a double bond
but possesses functional groups
such as hydroxyl, carboxyl, or amines, they can interact via condensation
• Example, monomer containing a reactive hydrogen from the
amine residue can react with another monomer containing a reactive hydroxyl
group (a residue of carboxyl group) to generate a new functional group (amide)
and water as a side product
• Nylon is prepared via condensation polymerization of a
diamine and diacid chloride
Examples of
condensation polymerization
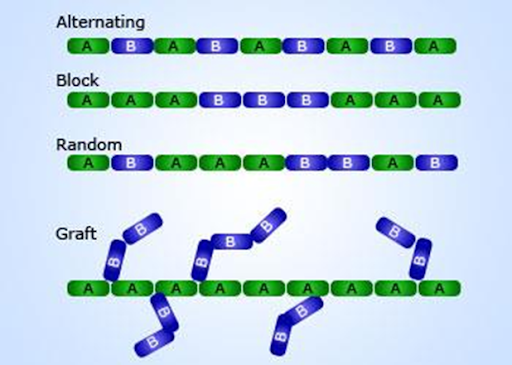
Classification
of Polymers
Polymers can be classified based on the following
Nature of monomers
1. Homopolymers
2. Copolymers
Arrangement of
monomers
1. Random
2. Graft
3. Block
Structure of polymer
1. Linear
2. Branched
3. Crosslinked
Thermal response
1. Thermoplastic
2. Thermosetting
3. Elastomer
Source
1. Natural
2. Semisynthetic
3. Synthetic
Copolymers
and Homopolymers
• If one monomer is involved, the process is called polymerization
and the product is a homopolymer
• Copolymerization refers to a polymerization reaction in
which more than one type of monomer is involved
• Generally, copolymerization includes two types of monomers
Other
Terminologies
Interpenetrating
Polymer Networks
Thermoplastic
and Thermoset Polymers
Thermoplastic
• Polymers with a linear or branched structure
• Can undergo melting
• The process of thermomelting and solidification can be
repeated indefinitely
Thermoset
• Cross-linked polymers
• There is no reversible melting and solidifying
• Once formed, it does not soften upon heating and
decomposes with further application of heat
Elastomers
• Rubbery polymers that can be easily stretched without
application of heat
• On releasing the applied stress, they return to original
dimensions
• Have low density of crosslinking
Biodegradable
and Nonbiodegradable polymers
• Based on the ability of the polymers to undergo
degradation in natural environment and biological systems
• Biodegradable –
slowly get degraded from the site of administration
• Non-biodegradable –
inert in the environment of use
Polymer
properties
Thermal
Physical
Mechanical
Thermal
• Melting point
• Glass transition temperature
Physical
• Molecular weight
• Molar volume
• Density
• Degree of polymerization
• Crystallinity of material
Mechanical
• Stretching
• Bending
• Hard or soft
• Response to
application of repeated load
Physical
Properties – Degree of Polymerization and Molecular Weight
• The degree of polymerization (DP)-n in a polymer molecule
is defined as the number of repeating units in the polymer chain − (−CH𝟐
− CH2−)−n
• The molecular weight of a polymer molecule is the product
of the degree of polymerization and the molecular weight of the repeating
unit
Average Molecular
weight
• The polymer molecules are not identical but are a mixture
of many species with different degrees of polymerization, that is, with
different molecular weights. Therefore, in the case of polymers we talk about
the average values of molecular weights
Significance
of polymer molecular weight
• The physical properties (such as transition temperature,
viscosity, etc.) and mechanical properties (such as strength, stiffness, and
toughness) depend on the molecular weight of polymer
• The lower the molecular weight, lower the transition
temperature, viscosity, and the mechanical properties
• Increased entanglement of chains with increased molecular
weight, the polymer gets higher viscosity in molten state, which makes the
processing of polymer difficult
Physical
Properties – Polydispersity Index (PDI) or Heterogeneity Index
• The dispersity
measures heterogeneity of sizes of molecules or particles in the mixture
• The mixture is called monodisperse if the molecules have
the same size, shape, or mass
• If the molecules in the mixture have an inconsistent size,
shape and mass distribution, the mixture is called polydisperse
The PDI is equal to or greater than 1
As the polymer chains approach uniform chain length, the PDI
approaches to unity
Physical
Properties – Polymer crystallinity
Semi-crystalline polymer
Crystalline
and amorphous polymers
Crystalline Polymer
• If the structure of polymer is linear, polymer chains can pack
together in regular arrays
Amorphous polymer
• In many cases, the structure of a polymer is so irregular
that crystal formation is thermodynamically infeasible
Physical
Properties – Polymer crystallinity
Amorphous O% ß
Polymer Crystallinity à >9O% Crystalline
• Lamellar crystalline form – the chains fold and make
lamellar structure arranged in the regular manner
• Amorphous form -the chains are in the irregular manner
• Tie Molecules – The lamellae are embedded in the amorphous
part and can communicate with other lamellae via tie molecules
Significance
of polymer crystallinity
Slow cooling + Simple
structural chains
â
Sufficient time is
available for crystallization to take place
â
High degree of Crystallinity
Rigid and have high
melting point, but their impact resistance is low
Examples:
polyethylene, and PET polyester
Amorphous polymers
are soft and have lower melting points
Solvent can
penetrate the amorphous part more easily than the crystalline part
Examples:
polystyrene and poly(methyl methacrylate)
Polymer
Crystallinity – Spherulites
• If the molten polymer is cooled down, then the crystalline
lamellae grow in radial direction from a nucleus along the three dimensions
leading to a spherical structure called spherulite
• The amorphous region is in between the crystalline
lamellae
• Due to highly ordered lamellae in the spherulite, it shows
higher density, hardness, tensile strength, and Young’s modulus
Thermal
Properties
Amorphous region in a polymer at different temperatures
Low temperatures
Polymer are in,
say, frozen state
The molecules can
vibrate slightly but are not able to move significantly. This state is referred
as the glassy state
The polymer is
brittle, hard and rigid analogous to glass.
Hence the name glassy
state
Higher temperatures
The polymer chains are able to wiggle around each other,
and the polymer becomes soft and flexible similar to rubber.
This state is called the rubbery state
Glass transition
temperature (Tg)
• The temperature at which the glassy state makes a
transition to rubbery state is called the glass transition temperature (Tg)
• The glass transition occurs only in the amorphous region,
and the crystalline region remains unaffected during the glass transition
• The glass transition temperature is the property of the
amorphous region of the polymer, whereas the crystalline region is characterized
by the melting point
• Glass transition temperature is the second order
transition, whereas the melting point is the first order transition
Glass
transition temperature and melting point
• The semi-crystalline polymer shows both the transitions corresponding
to their crystalline and amorphous regions
• Thus, the
semi-crystalline polymers have true melting temperatures (Tm) at which the
ordered phase turns to disordered phase
• The amorphous regions soften over a temperature range known
as the glass transition (Tg).
• Note: Amorphous polymers do not possess the melting point,
but all polymers possess the glass transition temperature
Factors
affecting melting point
• The polymer melting point Tm is increased if the double
bonds, aromatic groups, bulky or large side groups are present in the polymer
chain, because they restrict the flexibility of the chain
• The branching of chains causes the reduction of melting
point, as defects are produced because of the branching
Factors
affecting glass transition temperature
1. Intermolecular
Forces. Strong intermolecular forces cause higher glass transition
temperature
2. Chain Stiffness.
The presence of the stiffening groups (such as amide, sulfone, carbonyl,
p-phenylene etc.) in the polymer chain reduces the flexibility of the chain,
leading to higher glass transition temperature
3. Cross-Linking.
The cross-links between chains restrict rotational motion and raise the glass
transition temperature
4. Molecular Weight.
Tg is increased with the molecular weight
5. Plasticizers.
Plasticizers are low molecular weight and non-volatile materials added to
polymers to increase their chain flexibility. They reduce the intermolecular
cohesive forces between the polymer chains, which in turn decrease Tg
6. Pendant groups
• Bulky pendant
groups: the presence of bulky pendant group, such as a benzene ring, can
restrict rotational freedom, leading to higher glass transition temperature
• Flexible pendant
groups: the presence of flexible pendant groups, for example, aliphatic
chains, limits the packing of the chains and hence increases the rotational motion,
tending to less Tg value
Crystalline
or amorphous – Pharmaceutical perspective
• Polymer strength and stiffness increases with
Crystallinity as a result of increased intermolecular interactions
An amorphous polymer is preferred when the release of a drug
or an active material is intended
• Crystallinity increases the barrier properties of the
polymer.
• Small molecules like drugs or solvents usually cannot penetrate
or diffuse through crystalline domains
• Good barrier
properties are needed when polymers are used as a packaging material or as a coating
Mechanical
properties
1. Strength
2. Percentage
elongation to break (Ultimate Elongation)
3. Young’s Modulus
(Modulus of Elasticity or Tensile Modulus)
4. Toughness
5. Viscoelasticity
Strength
• Strength is the stress required to break the sample
• There are several types of the strength, namely,
Tensile (stretching of the polymer)
Compressional (compressing the polymer)
Flexural (bending of the polymer)
Torsional (twisting of the polymer)
Impact (hammering)
• The polymers follow the following order of increasing strength:
Linear < branched < cross-linked < network
Factors
Affecting the Strength of Polymers
• Molecular Weight:
In case of large molecular weight polymer, the chains become large and hence
are entangled, giving strength to the polymer
• Cross-linking: The
cross-linking restricts the motion of the chains and increases the strength of
the polymer
• Crystallinity: The
crystallinity of the polymer increases strength, because in the crystalline
phase, the intermolecular bonding is more significant
Percent
Elongation to Break (Ultimate Elongation)
• It measures the percentage change in the length of the
material before fracture
• It is a measure of ductility
Ceramics have very low (<1%)
Metals have moderate (1–50%)
Thermoplastic (>100%),
Thermosets (<5%)
Young’s
Modulus (Modulus of Elasticity or Tensile Modulus)
• Young’s Modulus is the ratio of stress to the strain in
the linearly elastic region
• Elastic modulus is a measure of the stiffness of the
material
Toughness
• The toughness of
a material is
given by the
area under a stress–strain curve
• The toughness measures the energy absorbed by the material
before it breaks
Mechanical
Properties
The rigid materials possess high Young’s modulus (such as brittle
polymers)
Ductile polymers also possess similar elastic modulus
Elastomers have low values of Young’s modulus and are
rubbery in nature
Viscoelasticity
• There are two types of deformations: elastic and viscous
Elastic deformation
• In the elastic deformation, the strain is generated at the
moment the constant load (or stress) is applied, and this strain is maintained
until the stress is not released
• On removal of the stress, the material recovers its
original dimensions completely, that is the deformation is reversible
Viscous deformation
• In viscous deformation, the strain generated is not instantaneous
and it is time dependent
• The strain keeps on increasing with time on application of
the constant load, that is, the recovery process is delayed
•When the load is removed, the material does not return to
its original dimensions completely, that is, this deformation is irreversible
Ideal
properties of polymer for pharmaceutical use
• Should be versatile and possess a wide range of
mechanical, physical and chemical properties
• Should be non-toxic and have good mechanical strength and
should be easily administered
• Should be inexpensive
• Should be easy to fabricate
• Should be inert to host and biodegradable
Advantages
of Polymers
• Polymers are more resistant to chemicals than their metal
counterparts
• Polymer parts do not require post-treatment finishing
efforts, unlike metal
• Polymer and composite materials are up to ten times
lighter than typical metals
• Polymer materials handle far better than metals in
chemically harsh environments.
This avoids problems associated with corroding metal
components
• In medical facilities
polymer and composite
materials are easier
to clean and sterilize than metal
• Polymers with desirable properties can be synthesized by
varying the monomers and their composition
Pharmaceutical
applications of polymers
• The desirable polymer properties in pharmaceutical
applications are
APPLICATION | PROPERTY |
Coating | Film forming |
Rheology Modifier | Thickening |
Controlled Release | Gelling |
Binding | Adhesion |
Controlled Release | pH-dependent solubility |
Taste Masking | Solubility in aqueous solvents |
Protection And Packaging | Barrier properties |
Binder
• In a traditional pharmaceutics area, such as tablet
manufacturing, polymers are used as tablet binders to bind the excipients of
the tablet
• Example: Poly(vinyl pyrrolidone) used as tablet
granulation
Packaging
materials for pharmaceutical products
• Flexible packages are made by the use of thin and flexible
polymer films
• When they are wrapped around a product, they can easily
adapt their shape to conform to the shape of the contents
• The thin, flexible films are usually produced from
cellulose derivatives, Poly(vinyl chloride)
(PVC), polyethylene, polypropylene, polyamide
(nylon), polystyrene, polyesters,
polycarbonate, poly(vinylidene chloride), and polyurethanes
• Heat sealable and are also capable of being laminated to
other materials
• Rigid packages such as bottles, boxes, trays, cups, vials,
and various closures are made from materials of sufficient strength and
inflexibility
• Widely used polymers are high-density polyethylene,
polypropylene, polybutene, poly(vinyl chloride), acrylic copolymers, polycarbonate,
nylon, and polyethylene terephthalate (PET)
• Biodegradable PET is preferred due to environmental
concerns, but it is expensive
Polyisoprene, ethylene propylene/dicylopentadiene copolymer,
styrene/butadiene copolymer, polybutadiene, silicone elastomers, and natural
rubber
Taste Masking
• Requirement for bitter drugs
• Applying polymer coatings
• It avoids direct contact of the bitter drug with the taste
buds
A water-soluble polymer
such as a
cellulose acetate, cellulose
butyrate, hydroxyethyl cellulose
is used in taste masking of bitter drug
Rheology
Modifiers
Natural sources
Starch, cellulose, alginate, carrageenan, collagen, gelatin,
guar gum, pectin, and xanthan gum
Synthetic
PVA, polyurethanes, acrylic polymers, CMC, HPMC, HMC
Gelling
• Acacia, alginic acid, bentonite, Carbopols (now known as carbomers),
carboxymethylcellulose, ethylcellulose (EC), gelatin, hydroxyethylcellulose,
hydroxypropyl cellulose, magnesium aluminum silicate, methylcellulose (MC),
poloxamers, polyvinyl alcohol (PVA), sodium alginate, and xanthan gum
Poly (vinyl
chloride)
Blood bag, hoses, and tubing
Contact
lenses
Hard contact lenses
Poly (methyl methacrylate)
Soft contact lenses
Poly (hydroxyethyl methacrylate)
Polystyrene
Water-Soluble
Synthetic Polymer
Poly (ethylene oxide)
à Coagulant, flocculent,
swelling agent
Poly (vinyl
pyrrolidone) à
Plasma replacement, tablet granulation
Poly (vinyl alcohol)
à Water-soluble
packaging, tablet binder, tablet coating
Poly (ethylene
glycol) à
Plasticizer, base for suppositories
Poly (isopropyl
acrylamide) and poly (cyclopropyl methacrylamide) à Thermogelling acrylamide
derivatives, its balance of hydrogen bonding, and hydrophobic association
changes with temperature
Water-Insoluble
Biodegradable Polymers
(Lactide-co-glycolide)
polymers à for
protein delivery
Starch-Based
Polymer
Sodium starch
glycolate à
Superdisintegrant for tablets and capsules in oral delivery
Starch à Glidant, a diluent in
tablets and capsules, a disintegrant in tablets and capsules, a tablet binder
Plastics
and Rubbers
Polycyanoacrylate à
Biodegradable tissue adhesives in surgery, a drug carrier in nano- and microparticles
Polychloroprene à
Septum for injection, plungers for syringes, and valve components
Polyisobutylene à
Pressure-sensitive adhesives for transdermal delivery
Silicones à
Pacifier, therapeutic devices, implants, medical grade adhesive for transdermal
delivery
Polystyrene à
Petri dishes and containers for cell culture
Poly (methyl methacrylate) à
Hard contact lenses
Poly (hydroxyethyl methacrylate) à Soft contact lenses
Poly (vinyl chloride) à
Blood bag, hoses, and tubing
Hydrocolloids
Carrageenan à
Modified release, viscosifier
Chitosan à
Cosmetics and controlled drug delivery applications, mucoadhesive dosage forms,
rapid release dosage forms
Pectinic acid à
Drug delivery
Alginic acid àOral
and topical pharmaceutical products; thickening and suspending agent in a
variety of pastes, creams, and gels, as well as a stabilizing agent for
oil-in-water emulsions; binder and disintegrant
Cellulose
based polymers
Hydroxypropyl methyl cellulose à Binder for tablet matrix and
tablet coating, gelatin alternative as capsule material
Hydroxyethyl and hydroxypropyl cellulose à Soluble in water and
in alcohol, tablet coating
Summary
• Historical evolution of polymers from guncotton to today’s
generation of modern polymers can be recalled
• ‘Poly’ means many and ‘mer’ means part
• Polymers are synthesized from monomers
• Polymers can be synthesized by addition or
condensationreactions
• Addition method is used when there are double bonds in monomers
• Condensation requires reactive groups in monomers
• Polymers are classified based on several factors like,
nature and arrangement of monomers, structure, source and thermal response of
polymer
• Physical properties of polymers include molecular weight, degree
of polymerization, crystallinity etc.
• Thermal properties include glass transition temperature
and melting point
• Mechanical properties include strength, elongation,
young’s modulus, toughness and viscoelasticity
• There are some specific properties required of polymers
for pharmaceutical use like, availability at affordable cost, non-toxicity,
biodegradability, etc.
• Specific advantages of polymers lend themselves to
specific applications in pharmaceutical and biomedical fields
• Polymers find applications in conventional and modified
drug delivery systems
• Polymers also find use in packaging and medical device fabrications
• As pharmaceutical excipients in the form of binders,
thickening agents, gelling agents, etc.
For PDF Notes Click on Download Button