Qualitative applications of UV Visible spectrophotometry
Objectives
After this session,
students will be able to
• Identify
possible electronic transitions in UV spectroscopy
• Enlist
the types of shifts observed in UV spectroscopy
• Identify
the significance of Absorption maxima
• Explain the solvent effects
Electronic
transitions in UV spectroscopy
UV spectrum
of Isoprene
Concept of Chromophore and Auxochrome
• Chromophore
is defined as any isolated covalently bonded group that shows a characteristic
absorption in the ultraviolet or visible region (200-800 nm).
Chromophores can
be divided into two groups
• a)
Chromophores which contain π
electrons and which undergo π→π*
transitions.
• Ethylenes
and acetylenes are the example of such chromophores.
• b)
Chromophores which contain both π
and nonbonding electrons. They undergo two types of transitions; π→π* and n→π*
• Carbonyl,
nitriles, azo compounds, nitro compounds etc. are the example of such
chromophores.
Auxochromes
• An
auxochrome can be defined as any group which does not itself act as a
chromophore but whose presence brings about a shift of the absorption band
towards the longer wavelength of the spectrum.
• –OH,-OR,-NH2,-NHR, -SH etc. are the
examples of auxochromic groups.
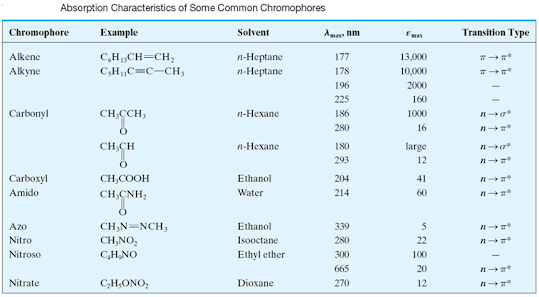
Chromophore
characteristics
Chromophore | Example | Excitation | λmax | Solvent |
C = C | Ethene | π → π* | 171 | Hexanes |
C = O | Ethanal | π → π* n → π* | 180 290 | Hexane |
N = O | Nitromethane | π → π* n → π* | 200 275 | Hexane |
Terminology for Absorption Shifts
Nature of the Shift | Descriptive Term |
To Greater Absorbance | Hyperchromic |
To Lesser Absorbance | Hypochromic |
To Longer Wavelength | Bathchromic or Red Shift |
To Shorter Wavelength | Hypsochromic or Blue Shift |
n àp* and p àp* Transitions
• Most
applications of absorption spectroscopy are based upon transitions for n or p electrons to the p* excited state
• The
energies required for these processes bring the absorption peaks into an
experimentally convenient spectral region (200 to 700 nm).
• Both
transitions require the presence of an unsaturated functional group to provide
the p orbitals.
• The
molar absorptivities for peaks associated with excitation to the n, p* state are generally low
and ordinarily range from 10 and 100 L cm-1 mol -1;
• Values
for p àp*
transitions, on the other hand, normally fall in the range between 1000 and
10,000.
Effect of Conjugation of Chromophores
• p electrons are considered
to be further delocalized by conjugation
• the
orbitals involve four (or more) atomic centers.
• The
effect of this delocalization is to lower the energy level of the p* orbital and give it less
antibonding character.
• Absorption
maxima are shifted to longer wavelengths as a consequence.
• Conjugation
of chromophores, has a profound effect on spectral properties.
• 1,3-butadiene,
CH2=CHCH=CH2, has a strong absorption band displaced to a
longer wavelength by 20 nm compared with the corresponding peak for an
unconjugated diene.
Absorption Involving d and f Electrons
• Most
transition-metal ions absorb in the ultraviolet or visible region of the
spectrum.
• For
the lanthanide and actinide series, the absorption process results from
electronic transition of 4f and 5f electrons
• For
elements of the first and second transition-metal series, the 3d and 4d
electrons are responsible.
Absorption by Lanthanide and Actinide Ions
• The
ions of most lanthanide and actinide elements absorb in the ultraviolet and
visible regions.
• Their
spectra consist of narrow, well-defined, and characteristic absorption peaks.
• The
transitions responsible for absorption by elements of the lanthanide series
involve the various energy levels of 4f electrons, while those of 5f electrons
of the actinide series
Absorption by Elements of the First and Second Transition-Metal Series
• The
ions and complexes of the first two transition series tend to absorb visible
radiation in one if not all of their oxidation states.
• The
absorption bands are often broad and are strongly influenced by chemical
environmental factors.
• The
spectral characteristics of transition metals involve electronic transitions
among the various energy levels of d orbitals.
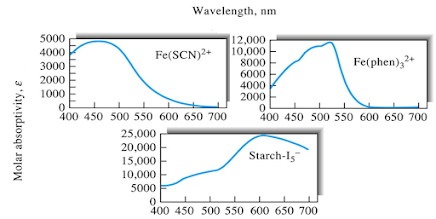
Charge-Transfer Absorption
• Species
that exhibit charge-transfer absorption are of particular importance because
their molar absorptivities are very large (emax
> 10,000).
• These
complexes provide a highly sensitive means for detecting and determining
absorbing species.
• Complexes
exhibit charge transfer absorption are called charge-transfer complexes.
• In
order for a complex to exhibit a charge-transfer spectrum, it is necessary for
one of its components to have electron-donor characteristics and for the other
component to have electron-acceptor properties.
• Absorption
of radiation then involves transfer of an electron from the donor to an orbital
that is largely associated with the acceptor.
APPLICATION OF ABSORPTION MEASREMENT TO QUALITATIVE ANALYSIS
• Methods
of Plotting Spectral Data: Several different types of spectral plots are
encountered in qualitative molecular spectroscopy. The ordinate is most
commonly percent transmittance, absorbance, log absorbance, or molar
absorptivity. The abscissa is usually wavelength or wavenumber, although
frequency is occasionally employed.
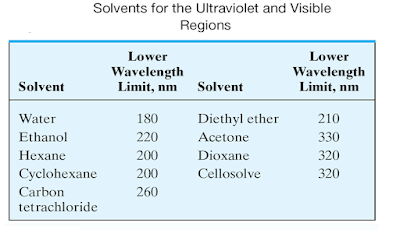
Solvent effects
• In choosing a solvent, consideration must be
given not only to its transparence, but also to its possible effects upon the
absorbing system.
• Polar
solvents such as water, alcohols, esters, and ketones tend to obliterate
spectral fine structure arising from vibrational effects
• spectra that approach those of the gas phase
are more likely to be observed in nonpolar solvents such as hydrocarbons.
• In
addition, the positions of absorption maxima are influenced by the nature of
the solvent.
• The
same solvent must be used when comparing absorption spectra for identification
purposes.
Summary
• Certain
electronic transitions are permitted and they will occur when chemical species
are exposed to light in UV and visible region
• Bathochromic
and hypso chromic shifts involve changes in absorption maxima
• Hyper
chromic and hypo chromic shifts involve changes in absorptivity values
• λmaxis characteristic of a substance
• Choice
of solvents depends on solvent effects
For PDF Notes Click on Download Button