β-lactam Antibiotics
Contents
• β-lactam antibiotics – penicillins
– History
• Structure
and properties of penicillin
• Structure
and activity relationships of penicillins
• Sensitivity
of penicillins – due to acid, β-lactamase
• Narrow
spectrum of activity of penicillins
• Extended
(broad) spectrum Penicillins
• Chemical
degradation of penicillins – various pathways
• Study
of individual penicillins including structures and specific uses
• β-lactamase inhibitors –
classification
• Mode
of action of β-lactamase
inhibitors
• Study
of individual compounds used as β-lactamase
inhibitors
Learning Objectives
At the
end of this lecture, student will be able to
Discuss the structure and properties of Penicillins
Explain the SAR of penicillins
Explain the sensitivity of penicillins towards acid and
enzymes
Describe the causes for narrow spectrum of activity of
Penicillins
Discuss the structural modifications to design extended
spectrum of penicillins
Discuss the chemical degradation of Penicillin
Discuss the structures, specific uses and side effects of
penicillins
Classify β-lactamase
inhibitors
Explain the mode of action of β-lactamase inhibitors
Discuss the structures, specific uses β-lactamase inhibitors
β-lactam Antibiotics
• Broad
spectrum of antibacterial action. The unequaled importance of β-lactam antibiotics in chemotherapy is due to
- Potent
lethal bactericidal action in the growth phase - Low
frequency of toxic and other adverse effects
MOA
• The
lethal antibacterial action is due to the selective imbibition of bacterial
cell wall synthesis. Specifically it inhibits the biosynthesis of peptidoglycan
which provides strength and rigidity to the cell wall.
• Pencillins
and cephalosporins acylate specific bacterial transpeptidases (Penicillin
binding proteins) and make them inactive
• PBP
1a &1b – transpeptidases involved in peptidoglycansynthesis associated with
cell elongation. Inhibition causes lysis
• PBP
2- transpeptidase involved in maintaining the rod shape in bacilli. Inhibition
causes ovoid or round forms which undergo lysis
• PBP
3- transpeptidase required for septum formation in cell division. Inhibition
results in formation of filamentous forms thast cannot separate
• PBP
4- carboxypeptidase responsible for the hydrolysis of the terminal peptide
bonds of crosslinking peptides. This cleavage of bond is required before
peptide crosslinkage. But inhibition of theses enzymes are not lethal
• The
various β-lactam antibiotics
differ in their affinities for the PBPs
β-lactam Antibiotics – Pencillins
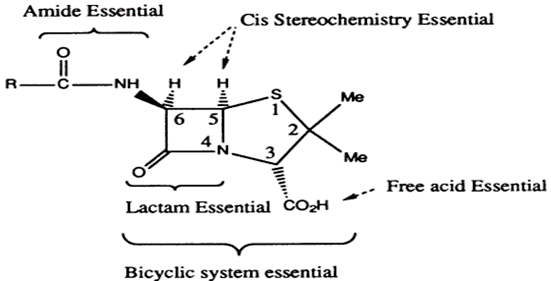
•
Structure
of Penicillin was established in 1945 by “ DOROTHY HODGKINS” by the use of X-ray
•
Penicillin
contains a bicyclic system consisting of a four-membered β-lactam ring fused to
a five membered thiazolidine ring
•
The
skeleton of the molecule suggests that it is derived from the amino acids
‘CYSTEINE’ and ‘VALINE’
•
The
over-all shape of the molecule is like a HALF OPEN BOOK.
•
It is a product of metabolism
•
The acyl
side chain (R) varies depending on the make-up of the fermentation media.
•
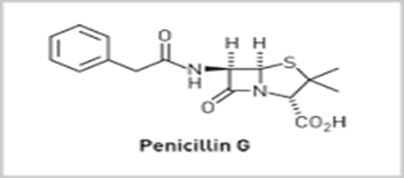
For
example, Corn steep liquor as the medium give penicillin G (R= Benzyl). This
was due to the high levels of Phenyl acetic acid (PhCH2COOH) present
in the medium.
Penicillin Analogues:-
•
One
method of varying the side chain is to add different carboxylic acids to the
fermentation medium.
•
For
example, Addition of phenoxy acetic acid (PhOCH2COOH) gives
Penicillin V.
Limitations:-
•
Only
acids of general formula ‘RCH2COOH’ can be added to the fermentation
medium, which restricts the variety of analogues.
•
Other
disadvantages – Tedious -Time-consuming process.
Properties of Penicillin G:-
•
Active
against Gram +ve bacilli (Staphylococci, meningitis and gonorrhea) and many
(but not all) Gram –ve Cocci.
•
Non-toxic
– The penicillins are amongst the safest drugs known to medicine.
•
Not
active over a wide range (or spectrum) of bacteria.
•
Ineffective
when taken orally since it breaks down in the acid condition of the stomach
•
Penicillin
G can only be administered by injection.
•
Sensitive
to all known β-lactamases. These are enzymes produced by penicillin-resistant
bacteria which catalyzes the degradation of penicillins.
•
Allergic
reactions are suffered by some individuals
There are several
problems associated with the use of Penicillin G; most serious ones
•
Acid-sensitivity
•
Sensitivity
to penicillinase
•
A narrow
spectrum of activity
•
The
purpose of making semi-synthetic penicillin analogues is to find compounds
which do not suffer from these disadvantages.
•
So the
study of ‘SAR’ and finding out the features important to its activity is vital
for making new effective analogues of ‘Penicillin G’.
STRUCTURE –ACTIVITY RELATIONSHIPS OF PENICILLINS
•
Fused
β-lactam and thiazolidine ring forming a bicyclic system (Penam). Bicyclic
system confers strain on the β-lactam ring
•
The free
carboxylic acid group is essential.
•
The
strained β-lactam ring is essential (increase strain, increase in activity,
increase instability)
•
The acyl
amino side chain is essential except for thienamycin
Thienamycin
•
Sulfur
is usual but not essential
•
The stereochemistry
of the bicyclic ring with respect to the acyl amino side chain is important –
Cis stereochemistry for the hydrogens
•
Very
little variation is tolerated by penicillin nucleus. Also, any variation which
can be made is restricted to the acyl amino side chain
Acid sensitivity of Penicillins:-
There are THREE
reasons for the acid sensitivity of ‘Penicillin G’
1) Ring Strain:
The bicyclic system in penicillin consists of a four-membered ring and a
five-membered ring. As a result, Penicillin suffers ‘LARGE ANGLE STRAIN and
TORSIONAL STRAIN”.
•
Acid
catalyzed ring openings relieves these strains by breaking open the more highly
stained four-membered lactam ring.
2) A highly
reactive β-lactam carbonyl group:
•
The
carbonyl group in the β-lactam ring is highly susceptible to nucleophiles and
does not behave like a normal tertiary amide which is usually quite resistant
to Nucleophilic attack.
•
This
difference in reactivity is due mainly to the fact that stabilization of the
carbonyl is possible in the tertiary amide, but is not possible in the β-lactam
ring.
•
The
β-lactam nitrogen is unable to feed its lone pair of electrons into the
carbonyl group since this would require the bi-cyclic rings to adopt an
impossibly strained flat system.
•
As a
result the lone pair of electrons is localized on the nitrogen and the carbonyl
group is far more electrophilic than one would expect for a tertiary amide.
•
The
normal tertiary amide is far less susceptible to nucleophiles since the
resonance structures reduce the electrophilic character of the carbonyl group
as follows:
3) Influence of
the acyl side chain [Neighboring group participation]
•
The
neighboring acyl group can actively participate in a mechanism to open up the
lactam ring. Thus penicillin G has self – destructive mechanism built into its
structure
Tackling the
problems of acid sensitivity:-
•
Nothing
can be done about the first two factors since the β-lactam ring is vital for
antibacterial activity.
•
Therefore,
only the third factor can be tackled i.e. reducing the effect or participation
of the neighboring group, by introducing a good electron withdrawing group
attached to the carbonyl group. This
electron-withdrawing group will draw the electrons away from the carbonyl
oxygen by inductive effect and reduce its tendency to act as a nucleophile
•
Influence
of the acyl side chain [Neighboring group participation]
•
For eg.,
Penicillin V, has an electronegative oxygen on the acyl side chain, so the
molecule has better acid stability than Penicillin G and is stable enough to
survive the acid in the stomach. Thus, it can be given orally
•
However,
Penicillin V is still sensitive to penicillinases and is slightly less active
than penicilln G
Other eg.,
AMPICILLIN
OXACILLIN
Penicillin sensitivity to β-lactamases:
•
β-lactamases
are enzymes produced by penicillin-resistant bacteria, which can catalyse the
reaction, in which the same ring opening and deactivation of penicillin which
occurred with acid hydrolysis.
β-lactamases
deactivation of penicillin
•
The
design of penicillinase-resistant penicillins involves the blocking of the
penicillin from reaching the penicillinase active site.
•
One way
to do this is to “place a bulky group on the side chain”. Thus bulky group act
as a ‘shield’ to prevent binding with the enzyme, penicillinase”.
•
But if
the shield is too bulky, then the steric shield also prevents the penicillin
from attacking the enzyme responsible for bacterial cell wall synthesis.
•
So the
Ideal ‘Shield’ will be that which would be large enough to ward off the
lactamase enzyme, but would be small enough to allow the penicillin to act on
the enzyme responsible for cell wall synthesis.
•
For eg.
Methicillin was the first semi-synthetic penicillin unaffected by penicillinase
and was developed to treat S.aureus infections, which was due to
virulent penicillin – resistant strains. Both the methoxy groups (ortho) on the
aromatic ring are important in shielding the lactam ring.
•
‘Methicillin’
is not an ideal drug, since there are no electron-withdrawing groups on the
side chain, it is acid sensitive, and so has to be injected.
•
It has ⅕
of the activity of Penicillin G against organisms sensitive to Penicillin G, it
shows poor activity against some streptococci, and it is inactive
against Gram –ve bacteria. This problem of acid sensitivity was solved by incorporating
into the side chain a five-membered heterocycle which was designated to act as
a steric shield and also to be electron-withdrawing.
•
These
compounds (Oxacillin, Cloxacillin and Flucloxacillin) are resistant to acid and
penicillinase and are useful against S. aureus infections.
•
The only
difference between the above three compounds is the type of halogen
substitution on the aromatic ring.
•
The
influence of these groups is found to be pharmacokinetic i.e. they influence
such factors as absorption of the drug and plasma protein binding.
•
For eg.
Cloxacillin is better absorbed through the gut wall than Oxacillin, whereas
flucloxacillin is less bound to plasma protein, resulting in higher levels of
the free drug in the blood.
•
These
also are inactive against Gram –ve bacteria.
•
Hence,
acid resistant penicillin would be the choice of the drug against an infection.
•
However,
if the bacteria proved resistance because of penicillinase enzyme, then the
therapy would be changed to penicillinase – resistance penicillin.
Narrow – spectrum of activity:-
•
Most
penicillins show a poor activity against Gram –ve bacteria.
There are several
reasons for this resistance.
Permeability barrier:-
•
It is
difficult for penicillins to invade a Gram –ve bacterial cell because of the
make of the cell wall.
•
Gram –ve
bacteria have a coating on the outside of their cell wall which consists of a mixture of fats, sugars
and proteins.
•
This
coating can act as barrier in various ways.
•
For eg.
The outer surface may have an overall –ve and +ve charge depending on its
constituent triglycerides.
•
An
excess of phosphatidyl Glycerol would result in an overall anionic charge
whereas an excess of lysyl phosphatidyl
Glycerol would result in an overall cationic charge.
•
Penicillin
has a free carboxylic acid which if ionized, would be repelled by the former
type of cell coat.
•
Alternatively,
the fatty portion of the coating may act as a barrier to the polar hydrophilic
penicillin molecule.
•
The only
way in which penicillin can pass such a barrier is through protein channels in
the outer coating-but most of these are usually closed.
•
High
levels of transpeptidase enzyme produced:-
•
The
transpeptidase enzyme is the enzyme attacked by penicillin. In some Gram –ve
bacteria, a lot of transpeptidase enzyme is produced and the penicillin is
incapable of inactivitating all the enzyme molecules present.
Modifications of the transpeptidase enzyme:-
•
A
mutation may occur which allows the bacterium to produce a transpeptidase
enzyme which is not antagonized by penicillin.
Presence of β-lactamase:-
•
β-lactamases
are enzymes which degrade penicillin. They are situated between the cell wall
and its outer coating.
Transfer of the β-lactamase enzyme:-
•
Bacteria
can transfer small portion of DNA from one cell to another through structures
called Plasmids. These are small pieces of circular bacterial DNA. If the
transferred DNA contains the code for the β-lactamase enzyme, then the
recipient cell acquires immunity.
Extended (Broad) Spectrum Penicillins
•
In order
to tackle the problem of narrow spectrum activity all the above factors has to
be considered. But the changes are confined only to the variations in the side
chain.
•
Introduction
of hydrophilic groups on the side chain (eg., Penicillin G) favour activity
against Gram +ve bacteria, but has poor activity against Gram –ve bacteria.
•
If
hydrophilic groups on the side chain have either little effect (eg., Penicillin
T) or cause a reduction of Gram +ve activity (eg., Penicillin N). But they lead
to an increase in activity against Gram –ve bacteria.
•
Enhancement
of Gram –ve activity is found to be greatest if the hydrophilic groups (e.g.,
NH2, OH, COOH) is attached to the carbon that is ‘Alpha’ to the carbonyl group
on the side chain.
•
Pencillins
which are active against, both Gram +ve and Gram –ve bacteria are known as
broad-spectrum antibiotics.
There are two
classes of broad-spectrum antibiotics
•
Both
classes have an ‘alpha’ hydrophilic group
•
Class I
broad spectrum antibiotics: Ampicillin and Amoxycillin
•
*where
hydrophilic group is –NH2 group:
•
Class II
broad spectrum antibiotics: Carbenicillin
•
*where
hydrophilic group is acid group,
Chemical
Degradation of Penicillins:-
• The
main cause of deterioration of penicillins is the reactivity of the strained
β-lactam ring, particularly by hydrolysis.
• The
course of the hydrolysis and the nature of the degradation products are
influenced by the pH of the solution.
• The
β-lactam carbonyl group of penicillin readily undergoes Nucleophilic attack by
water or hydroxide ion to form inactive ‘Penicilloic acid’ which is reasonably
stable in neutral to alkaline solutions but readily undergoes de-carboxylation
and further hydrolytic reactions in acidic solutions.
• Other
Nucleophiles as hydroxyl amines, alkyl amines and alcohols – open the β-lactam
ring to form the corresponding hydroxamic acids, amides and esters.
• In
strongly acidic solution (pH < 3), penicillin undergoes a complex series of
reactions forming a variety of inactive degradation products. The first step in
the rearrangement to the penicillanic acid. This process is initiated by
protonation of the β-lactam nitrogen,
followed by Nucleophilic attack of the acyl oxygen atom on the β-lactam
carbonyl carbon.
• Subsequent
opening of the β-lactam ring destabilizes the thiazolidine ring, suffers
acid-catalyzed ring opening to form penicillanic acid.
• Penicillanic
acid is very unstable and undergoes TWO major degradation pathways.
• ‘I
Path’ is hydrolysis of oxozolone ring to form unstable penamaldic acid, an
enamine easily undergoes hydrolysis to penicillamine (a major degradation
product) and penaldic acid.
• ‘II
Path’ involves a complex rearrangement of penicillanic acid to penillic acid.
• Penillic
acid (an imidazoline -2-carboxylic acid) readily decarboxylates and on
hydrolytic ring opening to form major end product penilloic acid.
• Penicilloic
acid (cannot be detected as intermediate), the major product formed. Weakly
acidic (neutral?) to alkaline hydrolytic conditions (also enzymatic
conditions), exists in equilibrium with penamaldic acid, undergoes
decarboxylation to give penilloic acid.
• The
third major product of the degradation is penicilloaldehyde formed by
decarboxylation of penaldic acid (a derivative of malonaldehyde) .
Individual
Compounds:
Penicillin G: [Benzyl
penicillin]
• Agent
of choice for the treatment of different kinds of bacterial infections than any
other antibiotic.
• Inactive
orally. But by combining antacids as calcium carbonate, aluminium hydroxide and
magnesium trisilicate or a strong buffer as sodium citrate and by giving large
doses, as it is poorly absorbed from intestinal tract, will increase the oral
activity of the drug.
• Water
soluble potassium or sodium salts are used orally and parenterally to achieve
high plasma concentration of pencillin G rapidly.
All pencillins should be administered with caution due to
allergic side effects
Penicillin V [Phenoxy
methyl Penicillin]
• It
is resistant to hydrolysis by gastric acid and it produces uniform
concentration in blood, (when administered orally).
• For
parenteral solution, potassium salt is usually used.
Cloxacillin:[3-(o-chlorophenyl)-5-methyl-4-isoxasolyl]
Penicillin sodium.
• Oxacillin,
cloxacillin and dicloxacillin are highly resistant to inactivation by
penicillinase. The steric effects of 3-phenyl and 5-phenyl groups prevent
binding to the B- lactamase active site.
• Prescence
of Cl in ortho position causes increase in activity due to increase in oral
absorption. It attains high plasma levels
Naficillin sodium
• [6-(2-ethoxy-1-naphthyl) penicillin sodium]
• Ethoxy
group and second ring of naphthalene group increase the stability against
penicillinase.
• Stable
to acid so it can be given by oral route
• Used
in infection caused solely by penicillin G-resistant staphylococci or
streptococci.
• Also
effective against pneumococci & group a – β-hemolytic streptococci.
• Should
be administered with care, due to its allergic side effects.
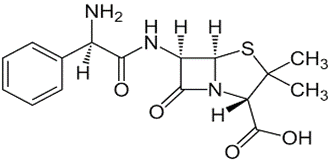
Ampicillin:
6-[D-α-aminophenyl acetamido] Penicillanic acid or D-α-amino benzyl penicillin
• It
has an anti-bacterial spectrum broader than that of Penicillin G.
• Active
against Gram –Ve organism that are susceptible to other penicillins
• Acid
resistant
• More
active against Gram –ve bacteria and enterococci than other penicillins
• Not
resistant to penicillinases
• Use:- Particularly useful for the treatment of
acute urinary tract infections caused by E.Coli or Proteus mirailis.
• It
is the agent of choice against Haemophilic influenzae infections
• Used
in combination with Probenicid for the treatment of Gonorrhea.
• Effective
in treating Salmonellosis and Shigellosis.
Amoxicillin:
6-[D-α-amino-p-hydroxy phenyl acetamido] Penicillanic acid (semi synthetic
penicillin)
• Its
antibacterial spectrum is same as that of ampicillin (resistant to acid,
susceptible to alkaline and β-lactamase hydrolysis).
• More
complete gastro-intestinal absorption to give higher plasma and urine levels.
• Less
diarrhea.
• Little or no effect of food on absorption.
β-lactamase inhibitors (Suicidal substrates)
• The
β-lactamase inhibitors, such as sulbactam and tazobactam and natural occurring
β-lactams, such as the thienamycins, inhibit both β-lactamases and interact
with penicillin binding protein (PBP) present in the bacterial cell wall.
• β-lactamase
inhibitors are given along with β-lactamase sensitive penicillin, so that they
competitively bind to the enzyme and protect the penicillin from destruction.
• β-lactamase
inhibitors are of 2 classes.
• Class. I – inhibitors:- have a heteroatom at
position 1
• Eg.
Clavulanic acid & sulbactam.
Clavulanic acid
Sulbactam
• Class. II – inhibitors:- do not have a heteroatom at position 1
Eg. Carbapenams – as Thienamycin
Mode of
action of β-lactamase inhibitors:-
• Inactivation
of β-lactamases is done by mechanism-based inhibitors, which act by reacting
with the enzyme in the same way as that of the substrate.
• An
acyl enzyme intermediate is formed by the reaction of the β-lactam with an
active-site serine hydroxyl group of the enzyme.
• For
normal substrates (Penicillins), the acyl enzyme intermediate readily undergoes
hydrolysis, destroying the substrate and freeing the enzyme to attack more
substrate.
• For
mechanism based-inhibitor, the acyl enzyme intermediate formed is diverted by
tautomerism to a more stable imine that undergo hydrolysis more slowly to
eventually free the enzyme (transient inhibitors).
• Because
these inhibitors are also substrates for the enzymes that they inactivate, they
are sometimes called as ‘Suicidal substrates’
Mechanism based inhibition of β-lactamase
Individual
Compounds of β-lactamase inhibitor:-
Clavulanate
potassium:-
• Clavulanic
acid is isolated from Streptomyces Clavuligeris
• *it
is 1-oxapenam
• *lacks
6-acylamino side chain
• *but
possess 2-hydroxyethylidene moiety at C-2.
• It
has very weak anti-bacterial activity
• It
is a potent inhibitor of S.aureus β-lactamase and plasmid-mediated
β-lactamases produced by Gram-ve bacilli.
• Combination
with Amoxacillin and potassium salt of Clavulanic acid (Augmentin) is intended
for the treatment of skin, respiratory,
ear and urinary tract infections caused by β-lactamase producing bacterial
strains- the oral bioavailability of both are the same
• It
is effective against strains which are
resistant to Amoxacillin alone
• It
is stable to acid
• Combination
of potassium clavulanate and ticarcillin sodium (extended–spectrum penicillin)
is recommended for Septicemia, lower respiratory tract infections and urinary
tract infections, bone and joint infections, skin & structure infections
caused by β-lactamase producing strains of S.aureus, Klebsiella, E.coli,
P.aeruginosa and other pseudomonas spp. Citrobacter spp. Enterobacter
spp. Serratia Marcescens, etc
Ticarcillin
disodium:-
Ticarcillin disodium ‘α-carboxy-3-thienyl penicillin’
Class II inhibitors – Carbepenams
• Class
of highly effective antibiotic agents commonly used for the treatment of severe
or high-risk bacterial infections. This class of antibiotics is usually
reserved for known or suspected multidrug-resistant (MDR) bacterial infections.
• Broadest
coverage of antibacterial activity
• Including
Gm+, and Gm- (especially drug resistant species), anaerobic coverage -Cover MSSA,
Enterococcus, streptococcus spp.
• Drugs
of choice for ESBL infections (ESBL-producing bacteria can’t be killed by many
of the antibiotics )
Carbapenams:
Thienamycin
• First
isolated from of Streptomyces Cattleya.
• ONLY
two structural features of thienamycins are shared with the penicillins and
cephalosporins.
• A
fused bicyclic ring system containing a β-lactam.
• An
equivalently attached 3-carboxyl group.
• The
presence of double bond between C-2 and C-3 in the bicyclic structure creates a
considerable ring strain and increases the reactivity of the β-lactam to ring
opening reactions.
• It
has a 1-hydroxylethyl gp at 6th position (not the acyl amino side
chain) & this is oriented to the α ring
• It
has broad spectrum antibacterial properties-active against most Gram+ &
gram- bacteria and resistant to activation by most β-lactamases (could be
because of hydroxyl ethyl side chain)
• It
is more susceptible to acid & alkaline hydrolysis coz the strain nature of
the fused ring system. Stable at pH between 6-7
Imipenam
• Very
stable to most β-lactamases. It is an inhibitor of β-lactamases from certain
Gram+ve & Gram-ve bacteria resistant to other β-lactamam antibiotics
• It
is used for the treatment of a wide variety of bacterial infections of the skin
& tissues, lower respiratory tract, bones & joints and genitourinary
tract infections.
• They
are also used for septicemia & endocarditis caused by β-lactamases
producing strains