Redox Titration

Learning Objectives
At the end of this lecture, the student will be able to
• Explain redox reaction, oxidation and reduction, oxidising and reducing agents
• Explain the Concepts of oxidation and reduction, Redox reactions
• Assign oxidation numbers
• Balancing redox reactions
• Calculate the equivalent weights of oxidising and reducing agents
• Redox Indicators
• Explain about the concept of permanganometry
• Explain the permanganate titrations
• Application of potassium permanganate method
• Explain the bromometric titrations
• Explain about the concept of Iodometry
• Explain about the concept of Iodimetry
• Discuss the applications of iodometry and iodimetry
• Explain the method of potassium Iodate titration
• Enumerate the Principles and applications of cerimetry
• Discuss the principle involved in bromometry.
• Explain the applications of bromometry.
• Discuss the principle involved in cerimetry.
• Explain the applications of cerimetry.
Reduction-Oxidation Reactions
Oxidation of Food: What a Waste!
• Fruits and Vegetables oxidised when left in open air
– Solution: Seal in plastic wrap
– More radical: Add lemon juice to the cut fruit
Oxidation of… People!
• Oxidation of nutrients causes increased activity of cells, leading to aging skin
– Solution: Beauty products?
What is a Redox Reaction?
• Redox – reduction + oxidation
• Both processes occur simultaneously
• Hence, one species is oxidised, another is reduced
• So, what is oxidation, and what is reduction?
• 3 different versions of the definition:
Redox
Oxidation | Reduction |
gain in oxygen | loss of oxygen |
loss of hydrogen | gain in hydrogen |
loss of electrons | gain of electrons |
Oxidation and Reduction
• In terms of Oxygen:
– Oxidation: Gain of oxygen in a species
• E.g. Mg is oxidized to MgO
– Reduction: Loss of oxygen in a species
• E.g. H2O is reduced to H2
– Note: It’s the gain or loss of O, not O2-
• In terms of Hydrogen:
– Oxidation: Loss of hydrogen in a species
• E.g. H2O is oxidised to O2
– Reduction: Gain of hydrogen in a species
• E.g. O2 is reduced to H2O2
– Note: It’s the gain or loss of H, not H+
• In terms of Electrons (OIL RIG: Oxidation Is Loss, Reduction Is Gain):
– Oxidation: Loss of electrons in a species
• E.g. Mg is oxidized to MgO (Mg from 12 electrons to 10 electrons in Mg2+)
– Reduction: Gain of electrons in a species
• E.g. O2 is reduced to H2O2 (O from 8 electrons to 9 electrons per O in O2 2-)
“LEO the lion goes GER.”
Losing Electrons is Oxidation
Gaining Electrons is Reduction
Oxidizing and Reducing agent
• An oxidising agent is a chemical species that causes the other reactant in a redox reaction to be oxidised, and it is always reduced in the process
• A reducing agent is a chemical species that causes the other reactant in a redox reaction to be reduced, and it is always oxidised in the process
• Remember:
– An oxidising agent is itself REDUCED when it oxidises something
– A reducing agent is itself OXIDISED when it reduces something
Example: 2Mg + O2 → 2MgO
– Mg is oxidised, and thus is the reducing agent
– O2 is reduced, and thus is the oxidising agent
List of Common Oxidizing and Reducing Agents
• Realise something?
– H2O2 is both an oxidising and a reducing agent!
– If a stronger oxidising agent is present, H2O2 is reducing
Variable Valence Elements
• Sulfur: SO4(+6), SO3(+4), S(0), FeS2(-1), H2S(-2)
• Carbon: CO2(+4), C(0), CH4(-4)
• Nitrogen: NO3- (+5), NO2- (+3), NO(+2), N2O(+1), N2(0), NH3(-3)
• Iron: Fe2O3(+3), FeO(+2), Fe(0)
• Manganese: MnO4 (+7), MnO2(+4), Mn2O3(+3), MnO(+2), Mn(0)
• Copper: CuO(+2), Cu2O(+1), Cu(0)
• Tin: SnO2(+4), Sn2+(+2), Sn(0)
• Uranium: UO2(+6), UO2(+4), U(0) 2+
• Arsenic: H3AsO4o (+5), H3AsO3o (+3), As(0), AsH3(-1)
• Chromium: CrO42-0(+6), Cr2O3(+3), Cr(0)
• Gold: AuCl4 (+3), Au(CN)2 (+1), Au(0)
Oxidation and Reduction
• In terms of Oxidation States:
– Oxidation: Gain in oxidation state in a species
• E.g. Mg is oxidized to MgO (Mg from 0 to +2 in Mg2+)
– Reduction: Gain of electrons in a species
• E.g. O2 is reduced to H2O2 (O from 0 to -1 in O2 )
• Note: Oxidation states are always written in +x or –x, never just x or x- (e.g. Oxidation State of Mg in MgO is +2, not 2 or -2)
General Guidelines for Identifying Redox Reactions
Oxidation | Reduction |
Complete loss of electrons (Ionic reaction) | Complete gain of electrons (Ionic reaction) |
Shift of electrons away from an atom in a covalent bond | Shift of electrons toward from an atom in a covalent bond |
Gain of oxygen | Loss of oxygen |
Loss of hydrogen by a covalent compound | Gain of hydrogen by a covalent compound |
Increase in oxidation number | Decrease in oxidation number |
Points to Remember
Oxidizing agent | Reducing agent |
Is itself reduced | Is itself oxidized |
Gains electrons | Loses electrons |
Causes oxidation | Causes reduction |
Assigning Oxidation Numbers
• An oxidation number is a positive or negative number assigned to an atom to indicate its degree of oxidation or reduction.
As a general rule, a bonded atom’s oxidation is the charge that it would have if the electrons in the bond were assigned to the atom of the more electronegative element
Rules for Assigning Oxidation Numbers
1. The oxidation number of a monatornic ion is equal in magnitude and sign to its ionic charge, For example, the oxidation number of the bromide ion (Br1-) is -1; that of the Fe3+ ion is + 3.
2. The oxidation number of hydrogen in a compound is + i, except in metal hydrides such as NaH, where it is – l.
3. The oxidation number of oxygen in a compound is – 2, except in peroxides, such as H202, where it is -1, and in compounds with the more electronegative fluorine, where it is positive.
4. The oxidation number of an atom in uncombined (elemental) form is o. For example, the oxidation number of the potassium atoms in potassium metal (K) or of the nitrogen atoms in nitrogen gas (N2 is o.
5. For any neutral compound, the sum of the oxidation numbers of the atoms in the compound must equal o.
6. For a polyatomic ion, the sum of the oxidation numbers must equal the ionic charge of the ion.
Balancing Redox Equations
Two main methods:
Oxidation-number change method
Half-Reactions
• Using the oxidation-number change method, involves balancing a redox equation by comparing the increases decreases in oxidation #s
• Fe2O3(s) + CO(g) → Fe(s) + CO2(g) (unbalanced)
• Step 1 – assign oxidation s to all the atoms in the equation
• Step 2 – Identify the atoms: oxidized and reduced
• Step 3 – Use one bracketing line to connect the atoms that undergo oxidation & another to connect reduced
• Step 4 – Make the total increase in oxidation # equal to the total decrease in oxidation # by using appropriate coefficients
• A half-reaction is an equation showing just the oxidation or just the reduction that takes place in a redox reaction
Example Problem
Balance the following redox equation.
Ce4+(aq) + Sn2+(aq) –> Ce3+(aq) + Sn4+(aq)
The reaction can be separated into a reaction involving the substance being reduced;
Ce4+(aq) + e– –> Ce3+(aq)
And the substance being oxidized;
Sn2+(aq) –> Sn4+(aq) + 2e–
We can see that the equations don’t balance; you must multiply the top equation by two, then add them to get
2 Ce4+(aq) + Sn2+(aq) –> 2 Ce3+(aq) + Sn4+(aq)
Simple Problem
The following redox reaction occurs in basic solution. Write complete, balanced equations for the individual half-reactions and the overall net ionic equation.
MnO4– (aq) + N2H4(aq) –> MnO2(s) + NO(g)
Oxidation Half Reaction:
8OH– (aq) + N2H4(aq) –> 2 NO(g) + 6 H2O(l) + 8 e–
Reduction Half Reaction:
3 e– + 2 H2O(l) + MnO4(aq) –> MnO2(s) + 4 OH– (aq)
Net Ionic Equation:
3 N2H4(aq) + 8 MnO4– (aq) –> 6 NO(g) + 8 MnO2(s) + 2 H2O(l) + 8 OH– (aq)
• Balance the following redox equations in acidic solution.
• Al(s) + I2(s) –> AlI3(s)
• KClO3(aq) + HNO2(aq) –> KCl(aq) + HNO3(aq)
Cl–(aq) + MnO2(s) –> Mn2+(aq) + Cl2(g)
• Balance the above redox equations in basic solution
Redox Titration
Equivalent weights of oxidising and reducing agents:
•In oxidation-reduction titrations, the equivalent weight of an oxidising agent or a reducing agent may be put as follows:
Eq weight of an oxidising and reducing agent= Molecular or ionic weight / No. of electrons gained or lost per molecule or ion of the substance
Equivalent weight in Oxidation-Reduction Reactions:
Equivalent weight of an oxidant or reductant can be defined as that weight of the substance, which reacts or contains 1.0078 g of available hydrogen or 8.0 g of available oxygen.
Equivalent weight can be calculated by:
a) Ion-electron balance method.
b) Oxidation number method.
a) Ion-electron balance Method: Ion electron balance method is based on following steps:
1. Ascertain the reactants and products of the reaction
2. Determine oxidizing agent. Write down partial equation for oxidizing agent
3. Determine reducing agent. Write down partial equation for reducing agent
4. Add both partial equations and cancel out common substances after multiplying both partial equations by suitable coefficient.
Electrochemistry Terminology
Electrochemical Cell– a device that converts electrical energy into chemical energy or vice versa
• Two Types:
Electrolytic cell
Converts electrical energy into chemical energy
Electricity is used to drive a non-spontaneous reaction
Galvanic (or voltaic) cell
Converts chemical energy into electricity (a battery!)
A spontaneous reaction produces electricity
• Conduction:
Metals:
Metallic (electronic) conduction — free movement of electrons
Solutions:
Electrolytic (ionic) conduction (or molten salts) — free movement of ions
Galvanic Cells
Galvanic Cells (batteries) — produce electrical energy e.g. a spontaneous reaction:
Cu(s) + Ag+ –> Cu2+(aq) + Ag (s)
(Ag metal will be deposited on a Cu wire dipped into aqueous AgNO3 solution)
In a galvanic cell, the half reactions are occurring in separate compartments (half-cells)
Electrochemical Conventions
Charges on the electrodes
Galvanic cell:
(+) cathode ~ reduction
(–) anode ~ oxidation
Electrolytic cell:
(+) anode ~ oxidation
(–) cathode ~ reduction
Cell Notation — summary of cell description
e.g.
Cu(s) | Cu2+(aq) || Ag+| Ag(s)
anode cathode
Cell Potential
Cell potential ~ Eºcell (an electromotive force, emf)
Units of Eºcell are volts: 1 volt = 1 joule/coulomb
Eºcell is a measure of the relative spontaneity of a cell reaction
Positive (+) Eºcell –> spontaneous reaction
Eºcell depends on:
– Nature of reactants
– Temperature — superscript º means 25 ºC
– Concentrations — superscript º means all conc are at 1.00 M and gases are at 1.00 atm
But, Eºcell is independent of amounts of reactants
Standard Reduction Potential:
The potential of a half-cell relative to a standard reference
Standard Cell Potential
Standard Reduction Potentials Eº (volts)
F2(g) + 2e– –> 2 F – (aq) + 2.87
Ag+(aq) + e– –> Ag(s) + 0.80
Cu2+(aq) + 2 e– –> Cu(s) + 0.34
2H+(aq) + 2 e– –> H2(g) 0.00
Zn2+(aq) + 2 e– –> Zn(s) – 0.76
Li+(aq) + e– –> Li(s) – 3.05
• Ease of Reduction ~ increases with Eº
e.g. F2 is easiest to reduce, Li+ is the hardest
• Standard Cell Potential ~ Eº cell can be determined from standard reduction potentials:
Eºcell = Eºred – Eºoxid
= [reduction potential of substance reduced] – [reduction potential of substance oxidized]
Standard Reduction Potentials
The Nernst Equation
E = Eº – [RT/nF]ln Q
The Nernst Equation shows the relationship between the standard cell potential (Eº) and the cell potential (E) under actual, non-standard conditions. this can be simplified at 25 ºC to:
E = Eº – (0.0592/n)log Q
Major use of the Nernst Equation:
• Determine concentrations from standard reduction potentials
• Use actual concentrations (i.e. Q) to calculate Ecell
Redox Indicators
• A Redox Indicator should be such that it produces a sudden change in the electrode potential in the vicinity of the equivalence point during a Redox Titration
• This is possible when the indicator itself is Redox Active i.e., capable of undergoing Oxidation or Reduction process which is a reversible one
• The oxidized and reduced form of the indicator should have a contrast difference in the colours
In oxd + ne = In red
• At potential E, the ratio of the concentration of two forms is given By the Nernst equation E T = E 0 + RT/nF ln [In oxd ]/ [I Red]
Types of Indicators:
1. Self-Indicator
2. External Indicator
3. Internal or Redox Indicator
4. Potentiometric Method
Self-Indicator:
• Self Indicator Potassium Permanganate is a good example for this category
• Many a times the titrant itself may be so strongly coloured that after the Equivalence point, a single drop of the titrant will impart a definite Pink colour at the END-Point of titration
• Ceric sulphate (Pale Yellow) and Iodine (Brown) are other examples of this category.
• The only disadvantage of Self-Indicators is that a slight over titration always occurs
External indicator:
• They are based on some visible reaction of the titrated substances with a suitable reagent, so that the end Point is marked by failure to elicit the reaction
• E.g.. Potassium Ferricyanide the titration of Ferrous iron by potassium dichromate. Drops of the solution removed to a spotting tile during titration will give a deep purssian blue colour with potassium ferricyanide because ferrous ions are still present
• At the end point, ferric ions are present and this does not give a colour with potassium ferricyanide
• E.g. – Starch in case of Iodimetry
• Ferrin Complex in case of Cerimetry
Internal or Redox Indicator:
• Redox Indicator are substances which have different colours in their Oxidized and Reduced form
• The reaction is Reversible
• Most of the Redox Indicators are Dyes, the reduced or leuco forms of which are Colourless
• E.g. Diphenylamine, Diphenylamine Sulphonate, Diphenyl Benzidine, Methylene Blue, Starch
Potentiometric Method:
• This is a Physico-chemical method which may be applied not only to those cases where suitable indicators are not available but also to those cases in which the visual indicator method fails or is limited accuracy. (e.g. for coloured solutions or very dilute solutions).
Types of Redox titrations
1. Titrations involving potassium permanganate as titrant: Permanganametry, example: Estimation of hydrogen peroxide
2. Titrations involving dichromate: Estimation of iron, chromium
3. Titration involving bromine as titrant: Bromometry, example: Estimation of isoniazid, phenol, liquified phenol
4. Titrations involving potassium iodate: Example of potassium iodide, weak iodine solution
5. Titration involving ceric ammonium sulphate/ sulphate as titrant: Cerrimetry, example: Estimation of ferrous sulphate, paracetamol tablets
6. Titrations involving Iodine:
a. Direct titration/Iodimetry Example: estimation of ascorbic acid
b. Indirect titrations/Iodometry Example:Estimation of copper sulphate, chlorinated lime
Permanganometry
• Potassium permanganate is a powerful oxidising agent
• Was first introduced into titrimetric analysis by F. Margueritte for the titration of iron(II)
• In acid solutions, the reduction can be represented by the following equation
• The standard reduction potential in acid solution, E0 has been calculated to be 1.51 volts; hence the permanganate ion in acid solution is a strong oxidising agent
• Sulphuric acid is the most suitable acid, as it has no action upon permanganate in dilute solution. With HCl, there is a likelihood of the reaction
• In the HCl , permanganate can oxidize Cl- to Cl2, which can be a source of positive errors as permanganate is consumed in this reaction. (E°red Cl2/Cl-)= +1.36V
Permanganate Titrations
KMnO4: Powerful oxidant that the most widely used
Eq. Wt. (=M/5): In strongly acidic solutions (1M H2SO4 or HCl, pH ≤ 1)
MnO4 – + 8H+ + 5e- = Mn2 + + 4H2O Eo= 1.51 V
Violet color colorless manganous
KMnO4 is a self-indicator.
In feebly acidic, neutral, or alkaline solutions (E=M/3)
MnO4 – + 4H+ + 3e- = MnO2 (s) + 2H2O Eo = 0.59 V
Brown manganese dioxide solid
In very strongly alkaline solution (2M NaOH or Ba (OH)2) (E=M/1)
MnO4 – + e- = MnO42 – Eo = 0.56 V
MnO4– + 4e- + 6F-+ 8H+ = [MnF6]3 – + 4H2O
E=M/4 (in HF or NH4HF2 Medium)
Preparation of 0.1 N potassium permanganate solution
1) Dissolve about 3.2 g of KMnO4 (mw=158.04) in 1000ml of water, heat the solution to boiling, and keep slightly below the boiling point for 1 hr. Alternatively, allow the solution to stand at room temperature for 2 or 3 days
2) Filter the liquid through a sintered-glass filter crucible to remove solid MnO2
3) Transfer the filtrate to a clean stoppered bottle freed from grease with cleaning mixture
4) Protect the solution from evaporation, dust, and reducing vapors, and keep it in the dark or in diffuse light. Preserve it in amber –coloured glass bottle
5) Standardise from time to time. If in time managanese dioxide settles out, re-filter the solution and restandardize it
Why potassium permanganate is secondary standard substance?
KMnO4 is not pure. Distilled water contains traces of organic reducing substances which react slowly with permanganate to form hydrous managnese dioxide. MnO2 promotes the auto decomposition of permanganate.
4 MnO4- +2H2O = 4 MnO2 +3O2 +4 OH-
Permanganate is inherently unstable in the presence of Mn+2 ions:
2MnO4- +3Mn2+ + 2H2O = 5 MnO2 + 4H+
Potassium permanganate solutions may be standardised using
Primary standards : arsenic(III) oxide or sodium oxalate
Secondary standards : metallic iron etc.
Standardization of potassium permanganate solution
Standardization by titration of sodium oxalate Na2C2O4.2H20 (primary standard) (Fowler and Bright) :
C2O42-= 2CO2 + 2 e- E°red = +0.77V
2KMnO4 +5 Na2(COO)2 +8H2SO4 = 2MnSO4 +K2SO4 +5Na2SO4 +10 CO2 + 8H2O
Standardization of potassium permanganate solution: in laboratory can also be done using oxalic acid
The reaction between oxalic acid and potassium permanganate can be represented as:
2KMnO4 + 5 H2C2O4 +3H2SO4 = 2MnSO4 +K2SO4 +10 CO2+ 8H2O
In ionic form the reaction can be represented as:
2MnO4– + 5 C2O4 2- + 16H+ = 2Mn2+ + 10 CO2 + 8H2O
Precaution:
• This titration is carried out in warm conditions (60 oC)
• The reaction at room temperature is slow because of the equilibrium nature of this reaction
• CO2 is highly soluble in water and thus heating removes all dissolved CO2 out of the solution driving the reaction in forward direction.
•Also at low temperature, the reduction of permanganate may not be complete producing Mn(III) (in the form [Mn(C2O4)3]3-).
• The formation of this species introduce errors in titrations as no. of electrons utilized here are different as compared to production of Mn2+
Standardization of potassium permanganate solution by Arsenic(III) oxide
•This procedure of H.A.Bright, which utilises As(III) oxide as a primary standardsand KI or potassium iodate (KIO3) as a catalyst for the reaction
• is convenient in practice and is a trustworthy method for the standardisation of permanganate solution
Chemical reaction involved
Application of Potassium Permanganate Method:
Hydrogen Peroxide Analysis:
• Because hydrogen peroxide decomposes in the presence of heat, light, or other catalysts, the quality of a hydrogen peroxide solution must be checked regularly to ensure its effectiveness
• The concentration of hydrogen peroxide can be analyzed by redox titration with potassium permanganate
2 KMnO4 + 3 H2SO4 + 5H2O2 à 2 MnSO4 + K2SO4 + 5O2 + 8H2O
Bromometry Titration
• Potassium bromate is a powerful oxidising agent which is reduced smoothly to bromide
• Potassium bromate is primary standard
• Potassium bromate can be used as oxidising agent for inorganic reducing agent determination
• For direct titration with potassium bromate are used specific redox indicators or various azo-dyes, which are destroyed by bromate ion surplus (disappearance colour)
• Mixture of potassium bromate and potassium bromide in acidified solution applies for back-titration of organic compounds
• This mixture produces bromine, which react with aryl radicals in organic compounds structure
• End point in bromometric back-titration is established with iodometric- like – titration with sodium thiosulfate, with starch serving as the indicator
Chemical Reaction involved in Bromometry titration
KBrO3: as titrant BrO3 – + 5Br– + 6H+ → 3Br2 + H2O
2I– + Br2 → I2 + 2Br–
I2 + 2 S2O3 2– → 2I– + 2S4O62–
Application of bromometry titration
Substitution reactions
BrO3 – + 5Br– + 6H+ → 3Br2 + H2O
Iodine as Oxidant
á EO | â EO |
I- can be oxidized by systems of higher oxidation potential | I- can be oxidized by systems of lower oxidation potential |
MnO4– /Mn2+ | Sn4+/Sn2+ |
Properties:
Iodometric method:
• Indirect titration
• Add KI to oxidizing agents
• Equivalent I2 is libarated and Titrated with Sodium thio sulphate(Na2S2O3)
• To determine oxidizing agents
Example: Copper sulphate
Iodimetric method
• Direct titration with I2
• To determine reducing agents
Example: Ascorbic acid
Systems having oxidation potentials near to that of iodine/iodide e.g AsO 3- /AsO 3-, Fe3+/Fe2+
Their reactions with Iodine is directed forward or backword by control of experimental conditions
i.e. Change in oxidation potential: by following factors
1- The pH of the medium
2- Addition of complexing agents
3- Addition of precipitating agents
Factors Affecting the Potential of I2/I- System:
1- Effect of pH:
The potential of: AsO 3-/AsO 3- = +0.57
I2/2I- = +0.54
To determine arsenite sample using Iodine the pH of the solution should be adjusted to 8.3 by adding NaHCO3
I2 + AsO 3- + H2O à 2I- + AsO 3- + 2H+
E AsO4 3- /AsO3 3- =Eo – 0.059 / 2 log [AsO33-] / [AsO43-][H+]2
↓ [H+] by addition of NaHCO3 ↓ the oxidation potential of AsO43- / AsO33- system NaHCO3 reacts with H+ giving CO2 and H2O shifting the reaction to the right and prevent reversibility
At higher pH if using NaOH, I2 reacts with OH- producing OI- so consuming more I2. Also OI- has oxidizing properties which differ than I2.
2- Effect of Complexing agents:
I2 + 2 e → 2I-
E= = Eo- 0.059/2 Log [I-]2 / [ I2]
When HgCl2 is added to the I2/I- system it forms [HgI4]2- Thus:
• Removing the I- ions from the share of the reaction
• Minimizing its concentration
• Increasing the ratio of I2 / [I-]2
• increasing the oxidation potential of I2 /2I- system
• So I2 could determine AsO3
3- Effect of precipitating agents
Fe(CN)6 3- + e → Fe (CN)6 4-
E = Eo – 0.059/1 log[Fe(CN)6]4-/[Fe(CN)6]3-
E° Ferri/Ferro= 0.36V
E° I 2/ 2I- = 0.54V
•Minimizing conc of ferrocyanide
• Increasing ferri/ferro potential
• So Ferri/Ferro system can oxidize I- to I2
To determine [Fe(CN)6]3- ion iodometrically; Zn2+ should be present: it precipitate Zn2[Fe(CN)6] ion
Titration methods:
Since iodine may be either reduced or produced by oxidation
Direct Iodimetric method | Indirect Iodometric method | |
Titrating agent | Iodine for determination of reducing agents | I- is added to oxidizing agents, the librated I2 is titr. with |
Indicator (Starch) | Added at the beginning of titr. | Added near the end of titr (when the brown color of I2 becomes pale) |
E.P. | permanent blue color | disappearance of blue color |
Detection of the end point in iodine titrations:
1- The use of starch:
• Starch is used in the form of colloidal Solu giving a deep blue adsorbtion complex with traces I2
• In exx I2 an irreversible blue adsorption complex is formed which is not changed
• Starch consists of βamylase and amylopectin I2 gives blue adsorption complex with β amylase.
• In strong acid medium: starch hydrolyses giving products which give with iodine non reversible reddish color masking the end point change
• Starch cannot be used in alcoholic solu. because alcohol hinders the adsorption of I2 on starch
• The sensitivity of the blue color decreases with temperature due to gelatinization of starch and volatility of Iodine
• Starch indicator solution must be freshly prepared when it stands decomposition takes place and its sensitivity is decreased. A preservative can be added
Starch-Iodine Complex
• Starch is the indicator of choice for those procedures involving iodine because it forms an intense blue complex with iodine. Starch is not a redox indicator; it responds specifically to the presence of I2, not to a change in redox potential.
• The active fraction of starch is amylose, a polymer of the sugar α- d-glucose.
• In the presence of starch, iodine forms I6 chains inside the amylose helix and the color turns dark blue
2- Use of organic solvent (CHCl3 or CCl4)
• In presence of alcohol or conc acids, organic solvents are recommended as indicators
• These solvents dissolve iodine to give intensely coloured purple solution, so that a trace of I2 gives an intense colour, and the end point will be the appearance Or disappearance of the colour in the organic solvent layer.
• I2 is soluble in CHCl3 or CCl4 90 times more than in H2O
• It is important that the mixture be shaken well near the end point in order to equilibrate the iodine between the aqueous and organic phases to enable aqueous S2O32- to react with I2 in CHCl3
Application of iodometry
Iodometry: Copper sulphate
CuSO4 + 2KI → CuI2 +K2SO4
2CuI2 →Cu2I2 +I2
I2 + Na2S2O3 → 2NaI + Na2S4O6
Cu2I2 + 2KSCN →2CuSCN + 2NaI
Indicator: Starch added towards the end point
Colour change: Blue to colorless
Iodimetry: Ascorbic acid
Indicator: Starch
End point: Blue to colorless
Potassium iodate titration
• Potassium iodate and potassium bromate are strong oxidizing agents than iodine
• The reaction between potassium iodate, an oxidizing agent and reducing agents such as potassium iodide or arsenic tri oxide in fairly acidic solutions
• Normally 0.1-2.0M hydrochloric acid, stops at the stage when iodate is reduced to iodine
IO3- +5I- +6H+ ó 3I2+3H2O
IO3- +5H3AsO3 +2H+ ó 3I2+5H3AsO4 +H2O
Later in 1903 Andrews showed that in the presence of a high concentration of hydrochloric acid (3-9M) iodateis reduced ultimately to iodine monochloride
IO3- + 6H+ +Cl- +4e ó ICl+3H2O
In hydrochloric acid solution, iodine monochloride forms a stable complex ion with chloride ion:
ICl + Cl- ó ICl2-
The overall half-cell reaction may therefore be written as:
IO3- + 6H+ +2Cl- +4e ó ICl2- +3H2O
End point detection in potassium iodate titration:
• Starch cannot be used, because at higher concentration of acidity it gets hydrolysed
• Hence a few ml of immiscible solvent such as carbon tetra chloride or chloroform, mat be added to the aqueous test solution contained in the glass stoppered flask
• The end point is marked by the disappearance of the last traces of violet colour, due to iodine, from the solvent: Iodine monochloride is not extracted by the organic layer and imparts yellow colour to the aqueous solution
• The extraction end point is very sharp
• The main disadvantage is the inconvenience of vigorous shaking with the extraction solvent in the stoppered vessel after each addition of the reagent near the end point
Ceric as titrant: Ce4+
•Ce4+ salts are strong oxidants in H2SO4
Ce4+ + e →Ce3+
Yellow Colorless
Properties
• Although it could be used as self-indicator it is preferable to use ferroin as indicator especially in case of det. of ferrous salts
• Ce4+ cannot be used in neutral or alkaline solution due to hydrolysis to hydrated ceric oxide
They have wide range of oxidising power but they don’t oxidise HCl even in presence of
Fe2+ salts
• Ceric salts are much more stable than MnO4-
• Ce4+ forms more stable complexes than Ce3+
Preparation and standardization of Ce4+ solution
Prepared from primary standard Ce(NO3)6 (NH4)2 in conc H2SO4 or in 72% HClO4. If using other salts it should be standardized
(1) Against arsenious trioxide:
2Ce4+ + H3 AsO3 + H2O→ 2Ce3+ + H3AsO4+ 2H+
(2) Against oxalate
2Ce4++ H2C2O4 ↔ 2Ce3+ + 2CO2 + 2H+
In both cases, the reaction is slow it requires heat to 50°C, using ICl as catalyst and ferroin indicator
Applications
(a) Direct titrations:
Determination of reducing agents: Fe2+, AsO33- , C2O42-,H2O22-,I–, Fe(CN)64- using ferroin indicator Color change from red to pale blue
For example: Hydrogen per oxide
H2O2 + 2Ce4+ → 2Ce3+ + 2H+ + O2
[Fe (CN)6]4-+ Ce4+ → Ce3++ [ Fe (CN)6]3-
Advantages:
estimation of hydrogen per oxide by Cerrimetry over permangometry
Better than MnO4 as it is less subject to interference of organic matter
It is preferable to be used instead of MnO4 in the determination of Fe2+ since we can use HCl
(b)Back itrations:
Determination of poly hydroxy alcohols, aldehydes, hydroxy acids.
Example: glycerol, citric acid
C3H8O3+8Ce4++3H2O à 3HCOOH+8Ce3++8H+
The excess Ce4+ is titrated against sodium oxalate or AsO33- indicator at 50oC.
Bromometry Titration
• Potassium bromate is a powerful oxidising agent which is reduced smoothly to bromide
• Potassium bromate is primary standard
• Potassium bromate can be used as oxidising agent for inorganic reducing agent determination
• Bromine solution (koppeschaars solution): It is produced by mixing excess of potassium bromate with an excess of potassium bromide in acidified solutions.
•specific redox indicators or various azo-dyes are used for direct titration with potassium bromate
• Mixture of potassium bromate and potassium bromide in acidified solution applies for back-titration of organic compounds.
• This mixture produces bromine, which react with aryl radicals in organic compounds.
• End point in bromometric back-titration is established with iodometric- like – titration with sodium thiosulfate, with starch as the indicator
Chemical Reaction involved in Bromometry titration
KBrO3: as titrant BrO3– + 5Br– + 6H+ → 3Br2 + H2O
2I– + Br2 → I2 + 2Br–
I2 + 2 S2O3 2 –→ 2I– + 2S4O6 2-
Application of bromometry titration
OTHER PHENOLIC COMPOUNDS ASSAYED BY BROMOMETRY
CERIMETRY
Ceric as titrant: Ce4+
•Ce4+ salts are strong oxidants in H2SO4
Ce4+ + e →Ce3+
Yellow Colorless
Properties
• Although it could be used as self-indicator it is preferable to use ferroin as indicator especially in determination of ferrous salts
• Ce4+ cannot be used in neutral or alkaline solution due to hydrolysis to hydrated ceric oxide They have wide range of oxidising power but they don’t oxidise HCl even in presence of Fe2+ salts
• Ceric salts are much more stable than MnO4
• Ce4+ forms more stable complexes than Ce3+
Preparation and standardization of Ce4+ solution
Prepared from primary standard Ce(NO3)6 (NH4)2 in conc H2SO4 or in 72% HClO4. If using other salts it should be standardized
(1) Against arsenious trioxide:
2Ce4+ + H3 AsO3 + H2O→ 2Ce3+ + H3AsO4+ 2H+
(2) Against oxalate
2Ce4++ H2C2O4 ↔ 2Ce3+ + 2CO2 + 2H+
In both cases, the reaction is slow it requires heat to 50°C, using ICl as catalyst and ferroin indicator
Applications
(a)Direct titrations:
Determination of reducing agents: Fe2+, AsO33-, C2O42-, H2O2, I–, Fe(CN)64- using ferroin indicator
Color change from red to pale blue
For example: Hydrogen peroxide
H2O2 + 2Ce4+ → 2Ce3+ + 2H+ + O2
[ Fe (CN)6]4-+ Ce4+ → Ce3++ [ Fe (CN)6]3-
Advantages: estimation of hydrogen per oxide by Cerimetry over permangometry
Ø Better than MnO4– as it is less subject to interference of organic matter
Ø It is preferable to be used instead of MnO4– in the determination of Fe2+ since we can use HCl
(b)Back Titrations: Determination of poly hydroxy alcohols, aldehydes, hydroxy acids.
Example: glycerol, citric acid
C3H8O3+8Ce4++3H2Oà 3HCOOH+8Ce3++8H+
The excess Ce4+ is titrated against sodium oxalate or AsO33- using ICl as catalyst and ferroin as indicator at 50oC.
Summary
• Oxidation means loss of hydrogen, gain of oxygen, loss of electrons
• Reduction means gain of hydrogen, loss of oxygen, gain of electrons
• Redox reactions are those reaction in which oxidation and reduction occurs simultaneously.
• Examples of oxidising agent and reducing agents are studied.
• Redox reactions include reactions which involve the loss or gain of electrons.
• The reactant giving away (donating) electrons is called the reducing agent (which is oxidized)
• The reactant taking (accepting) electrons is called the oxidizing agent (which is reduced)
• Both oxidation and reduction happen simultaneously, however each is considered separately using ion-electron equations
• Two main methods for assigning oxidation number: Oxidation-number change method and Half-Reactions
• Eq weight of an oxidising and reducing agent= Molecular or ionic weight/No. of electrons gained or lost per molecule or ion of the substance
• Electrochemical Cell – a device that converts electrical energy into chemical energy or vice versa
• Nernst Equation= Eº – [RT/nF]ln Q
• Different types of Redox Indicators are studied.
1. Self Indicator
2. External Indicator
3. Internal or Redox Indicator
4. Potentiometric Method
Examples of redox indicators are studied.
• Types of redox titration: Bromometry, permanganometry, cerimetry, Iodimetry, Iodometry
• Permanganometry: Potassium permanganate which is strong oxidizing agent is used as titrant in acidic media
• Application of Permanganometry: hydrogen per oxide
• Bromometry: Potassium bromide and potassium bromate in acidic media liberates bromine which is used as titrant for estimating reducing substances
• Application of bromometry: Phenolic compounds
• Iodine titrations: Iodimetry, Iodometry
Factors effecting iodimetric titrations:
1. pH
2. Complexing agents
3. precipitating agents
Indicators used in this titration: Starch, Organic solvents like chloroform, carbon tetra chloride
• Bromometry: Potassium bromide and potassium bromate in acidic media liberates bromine which is used as titrant for estimating reducing substances
• Application of bromometry: Phenolic compounds
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy




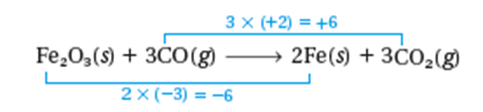















download