Major Extra and Intracellular electrolytes

Major Extra and Intracellular electrolytes
Learning Objectives
At the end of this lecture, the student will be able to:
• Define electrolytes
• List, describe and compare the body fluid compartments
• Compare the compositions of intracellular and extracellular fluids
• Identify the hormones that play important roles in regulating fluid and electrolyte balance
• Describe the function of Electrolytes
• List the normal concentration of cations and anions present in the body fluid compartments
• Describe the physiological role of sodium ion and potassium ion
• Describe the physiological role of:
Chloride ion
Bicarbonate ion
Phosphate ion
Calcium ion
Magnesium ion
• Calculate the amount of electrolyte in terms of milliequivalnet
• Discuss the physiological role of acid and bases and buffers
• Describe the different buffer system present in the body
• Define metabolic acidosis and metabolic alkalosis
• Explain the monograph analysis of:
Sodium Chloride
Potassium chloride
Calcium Gluconate
Oral dehydration salts
Introduction
Electrolytes: Substances whose molecules dissociate into ions when they are placed in water
Medically significant / routinely ordered electrolytes include:
CATIONS (+)
ANIONS (-)
Cation: Positively Charged particles
Sodium (Na+)
Potassium (K+)
Calcium (Ca++)
Magnesium (Mg++)
Anion: Negatively charged particles
Chloride (Cl-)
Bicarbonate (HCO3-)
Phosphate (HPO4-)
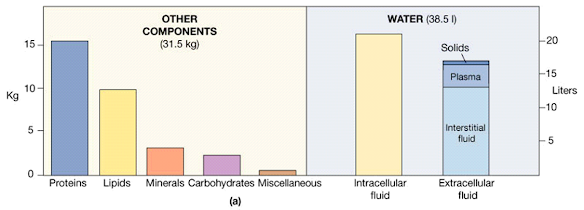
The Composition of the Human Body
Body Fluids: Introduction
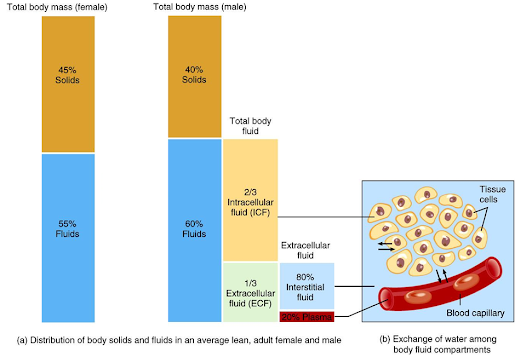
• Total amount of fluid in the human body is approximately 70% of body weight
Body fluid has been divided into two compartments:–
Intracellular fluid (ICF)
• Inside the cells
• 55% of total body
Extracellular fluid
• Outside the cells
• 45% of total water body water
Extracellular fluid includes
Interstitial fluid
Present between the cells
Approximately 80% of ECF
Plasma
Present in blood
Approximately 20% of ECF
Also includes
Lymph
Synovial fluid
Aqueous humor
Cerebrospinal fluid
Compartment separation:
Barriers separate ICF, interstitial fluid and plasma:
Plasma membrane:
Separates ICF from surrounding interstitial fluid
Blood vessel wall:
Separate interstitial fluid from plasma
Body Fluid Compartments in human system
Composition of body fluids
|
Inorganic substances |
Organic substances |
|
• Sodium • Potassium • Calcium • Magnesium • Chloride • Phophate • Sulphate |
• Glucose • Amino acids • Fatty acids • Hormones • Enzymes
|
Methods of fluid & electrolyte movement
• Diffusion
• Osmosis
• Active Transport
• Filtration
Diffusion
• Process by which a solute in solution moves
• Involves a gas or substance
• Movement of particles in a solution
• Molecules move from an area of higher concentration to an area of lower concentration
• Evenly distributes the solute in the solution
For eg: diffusion of CO2 and O2 at respiratory epithelia
Osmosis
• Movement of the solvent or water across a membrane
• Involves solution or water
• Equalizes the concentration of ions on each side of membrane
• Movement of solvent molecules across a membrane to an area where there is a higher concentration of solute that cannot pass through the membrane
Active transport system
• Moves molecules or ions uphill against concentration & osmotic pressure
• Hydrolysis of adenosine triphosphate (ATP) provides energy needed
• Requires specific “carrier” molecule as well as specific enzyme (ATPase)
• Sodium, potassium, calcium, magnesium, plus some sugars, & amino acids use it
Filtration
• Movement of fluid through a selectively permeable membrane from an area of higher hydrostatic pressure to an area of lower hydrostatic pressure
• Arterial end of capillary has hydrostatic pressure > than osmotic pressure so fluid & diffusible solutes move out of capillary
Hormones involved Fluid balance and electrolyte balance
Fluid balance and electrolyte balance are mediated by three hormones:
• Antidiuretic hormone (ADH)
• Aldosterone
• Renin
ADH (Antidiuretic Hormone)
• Produced in hypothalamus; water conservation hormone
• Stored in posterior pituitary gland
• Acts on renal collecting tubule to regulate reabsorption or elimination of water
• If blood volume decreases, then ADH is released & water is reabsorbed by kidney. Urine output will be lower but concentration will be increased.
Aldosterone
• Produced by adrenal cortex
• Released as part of RAA mechanism
• Acts on renal distal convoluted tubule
• Regulates water re absorption by increasing sodium uptake from the tubular fluid into the blood but potassium is excreted
• Responsible for re absorption of sodium & water into the vascular compartment
RENIN
• Released by kidneys in response to decreased blood volume
• Causes angiotensinogen (plasma protein) to split & produce angiotensin I
• Lungs convert angiotensin I to angiotensin II
• Angiotensin II stimulates adrenal gland to release aldosterone & causes an increase in peripheral vasoconstriction
Electrolyte Functions in general:
• Volume and osmotic regulation
• Myocardial rhythm and contractility
• Cofactors in enzyme activation
• Regulation of ATPase ion pumps
• Acid-base balance
• Blood coagulation
• Neuromuscular excitability
• Production of ATP from glucose
Normal plasma range of Electrolytes
Cations
Major cations inside the cell (ICF) include
• Sodium (Na)
• Potassium (K)
• Magnesium (Mg)
Major cations outside the cell (ECF) include
• Sodium (Na)
• Potassium (K)
• Calcium (Ca)
• The concentration of cations inside the cell and outside the cell differs as shown in figure on the right e.g. there is much higher concentration of potassium in the ICF than in the ECF, there is much higher concentration of sodium in the ECF than in the ICF.
Anions
Major Anions inside the cell (ICF) include
• Chloride (Cl)
• Proteins
• Phosphates (HPO4)
• Bicarbonate (HCO3)
• SO4
Major Anions outside the cell (ECF) include
• Chloride (Cl)
• Proteins
• Phosphates (HPO4)
• Bicarbonate (HCO3)
• SO4
• The concentration of anions inside the cell and outside the cell differs as shown in figure on the right e.g. there is much higher concentration of proteins and Phosphate in the ICF than in the ECF, there is much higher concentration of chloride in the ECF than in the ICF
Cations and Anions in Body Fluids
• Despite the differences in the concentration of specific substances, the ICF and ECF osmotic concentrations are identical
• If the cell membrane were freely permeable, diffusion would continue until these ions were evenly distributed across the membrane
Sodium (Na+)
• Range 135 – 145 mEq /L in serum
• Total body volume estimated at 40 mEq/kg
• 1/3 fixed to bone, 2/3 extracellular and available for Trans membrane exchange
• Normal daily requirement 1-2 mEq/kg/day
• Chief extracellular cation about 90%
Food Sources
|
High Sodium |
Low Sodium |
|
Bacon Corned beef Ham Catsup Potato chips Pickles Olives Soda crackers Tomato juice Beef cubes Dill Decaffeinated coffee |
Fruit Fresh Frozen canned Unsalted grains Pastas Oatmeal Popcorn Shredded wheat Fresh meats
|
Sodium function
• Transmission and conduction of nerve impulses
• Responsible for osmolality of vascular fluids
• Regulation of body fluid levels
• Sodium shifts into cells and potassium shifts out of the cells (sodium pump)
• Assists with regulation of acid-base balance by combining with Cl- or HCO3 to regulate the balance
Clinical Features:
• IF serum sodium ions is < 135 mmol/L Hyponatremia
•Increased Na+ loss
• Aldosterone deficiency
• Addison’s disease (hypo-adrenalism, result in desearsed aldosterone)
• Diabetes mellitus
• In acidosis of diabetes, Na is excreted with ketones
• Potassium depletion
• K normally excreted, if none, then Na
• Loss of gastric contents
Clinical manifestations of Hyponatremia
• Neurological symptoms: Lethargy, headache, confusion, apprehension, depressed, reflexes, seizures and coma
• Muscle symptoms: Cramps, weakness, fatigue
• Gastrointestinal symptoms: Nausea, vomiting, abdominal cramps and diarrhoea
Hypernatremia: Serum Sodium exceeds 145 meq/liter
Clinical Features: Hypertonic IV solution
• Oversecretion of aldosterone
• Loss of pure water
• Long term sweating with chronic fever
• Respiratory infection → water vapor loss
• Diabetes – polyuria
• Insufficient intake of water ( hypodipsia hypodipsia)
• Cushing’s syndrome – opposite of Addison’s
Clinical manifestations of Hypernatremia
• Thirst
• Lethargy (Lack of energy)
• Neurological dysfunction due to dehydration of brain cells
• Decreased vascular volume
Treatment of Hypernatremia
• Lower serum Na+
• Isotonic salt -free IV fluid
• Oral solutions preferable
Potassium ions( K+)
Potassium normal values
Serum (adults) – 3.5 – 5.3 mEq/L
Newborns slightly higher – 3.7 – 5.9 mEq/L
• Range 3.5 – 5.0 mEq mEq/L in serum
• Total body volume estimated at 50 mEq/kg
• 98% intracellular concentration of 150 mEq mEq/L
• Extracellular concentration of 70 mEq/L
• Normal daily requirement 0.5 – 0.8 mEq/kg/day
• Chief intracellular cation
Potassium Function:
• Most abundant intracellular cation
Necessary for transmission and conduction of nerve impulses
• Maintenance of normal cardiac rhythm
Necessary for smooth and skeletal muscle contraction
Food sources – veggies, fruits, nuts, meat
Causes of Hypokalemia
• Increased K+ loss
• Chronic diuretics
• Acid/base imbalance
• Trauma and stress
• Increased aldosterone
• Redistribution between ICF and ECF
• Hepatic disease
• Acute alcoholism
Clinical manifestations of Hypokalemia
• Neuromuscular disorders
• Weakness, flaccid paralysis, respiratory arrest, constipation
• Dysrhythmias: appearance of U wave
• Postural hypotension
• Cardiac arrest
Treatment: Increase K+ intake, but slowly, preferably by foods
Hyperkalemia
• Serum K+ > 5.5 mEq / L
• Check for renal disease
• Massive cellular trauma
• Insulin deficiency
• Addison Addison’s disease
• Potassium sparing diuretics
• Decreased blood pH
• Exercise causes K+ to move out of cells
Treatment of Hyperkalemia
If time, decrease intake and increase renal excretion
• Insulin + glucose
• Bicarbonate
• Ca++ counters effect on heart
Chloride Cl-
– The normal adult value for chloride is 97-107 mEq/L.
– Chloride is the major extracellular anion
– Moves relatively easily between ECF and ICF because most plasma membranes contain Cl
– Leakage channels and antiporters
– Can help balance levels of anions in different fluids
• Chloride shift in RBCs
– Regulated by
• ADH – governs extent of water loss in urine
• Processes that increase or decrease renal reabsorption of Na+ also affect reabsorption of Cl-
• Chloride is an important electrolyte
• Maintains body’s metabolism
• Maintains water balance, acid-base balance, aids in digestion (hydrochoric acid) & osmotic pressure (with Na and H20) Combines with Na to form salts
• Regulated by kidneys
Source of foods – Citrus fruits, Salt
Clinical features: Hyperchloremia
Hyperchloremia (increased plasma Cl concentration)
• Serum level > 106mEq/
Causes of hyperchloremia
May include:
• Loss of body fluids from prolonged vomiting, diarrhea, sweating or high fever (dehydration).
• High levels of blood sodium.
• Kidney failure, or kidney disorders
• Diabetes insipidus or diabetic coma
• Drugs such as: androgens, corticosteroids, estrogens, and certain diuretics
Treatment: restore fluid & electrolyte balance
Symptoms of Hyperchloremia
• Many people do not notice any symptoms of hyperchloremia, unless they are experiencing very high or very low levels of chloride in their blood
• Dehydration, fluid loss, or high levels of blood sodium may be noted.
• Diarrhea, or vomiting when suffering from hyperchloremia
• Arrhythmias, decreased cardiac output, muscle weakness, LOC changes, Kussmauls’s respirations
Clinical features: Hypochloremia
Hypochloremia (decreased plasma Cl concentration)
Serum level 96mEq/L
Results from prolonged vomiting & suctioning
Is observed in salt-losing nephritis as associated with chronic pyelonephritis
Treatment: diet/IV therapy
Causes of hypochloremia may include:
•Loss of body fluids from prolonged vomiting, diarrhea, sweating or high fevers
•Drugs such as: bicarbonate, corticosteroids, diuretics, and laxatives
Symptoms of Hypochloremia
• Dehydration, fluid loss, or high levels of blood sodium may be noted
• Diarrhea, or vomiting
• Metabolic alkalosis
• Nerve excitability
• Muscle cramps
• Twitching
• Hypoventilation
• Decreased BP if severe
Phosphate PO4-
• Principal anion of intracellular fluid compartment
• Plasma 1.7-2.6 mEq/liter
• Phosphate (H2PO4 -, HPO42-, PO4 3-)
• Most (85%) is stored in bone as calcium salts
• Also combined with lipids, proteins, carbohydrates, nucleic acids (DNA and RNA), B vitamin synthesis
• High energy phosphate transport compound
• Important acid-base buffer in body fluids
Source of foods: Dairy product, meats, poultry, fish, egg, nuts.
Function of phosphate:
• Involved in acid–base buffering system, ATP production, and cellular uptake of glucose
• Maintenance requires adequate renal functioning
• Essential to muscle, RBCs, and nervous system function
Clinical features: Hypophosphatemia
• Serum level < 1.8mEq/L
• Results from decreased intestinal absorption and increased excretion
• S/S bone & muscle pain, mental changes, chest pain, respiratory failure
Treatment: Diet/ IV therapy
Management
– Oral supplementation
– Ingestion of foods high in PO43-
– IV administration of sodium or potassium phosphate
Clinical features: Hyperphosphatemia
• Serum level> 2.6mEq/L
• Results from renal failure, low intake of calcium
• S/S: neuromuscular changes (tetany), EKG changes, parathesia-fingertips/mouth
Treatment:
Diet; hypocalcemic interventions
Medications: phosphate binding
• The body can tolerate hyperphosphatemia fairly well BUT the accompanying hypocalcemia is a larger problem!
Treatment:
– Identify and treat underlying cause
– Restrict foods and fluids containing PO43-
– Adequate hydration and correction of hypocalcemic conditions
Bicarbonate HCO3-
• Bicarbonate 22-28 mEq/L
• Second most prevalent extracellular anion
• Principle buffer of body pH. (Extracellular)
• Neutralizes acids
• Plays important role in acid / base balance
• Acts as chemical sponge to soak up Hydrogen ions
• (Acidic metabolic waste) for every one Hydrogen ion twenty bicarbonate ions are released to maintain balance
Regulation:
Bicarbonate is regulated by secretion/reabsorption of the renal tubules
Acidosis: ↓ renal excretion
Alkalosis: ↑ renal excretion
Kidney regulation requires the enzyme carbonic anhydrase – which is present in renal tubular cells & RBCs carbonic anhydrase
Reaction: CO2 + H2O ⇋ H2CO3 → H++ HCO3–
Clinical Significance:
• Alterations of HCO3- and CO2 dissolved in plasma are characteristic of acidbase imbalance
• When acid-base imbalance is suspected, evaluation of blood gases and pH is required
– Increase in CO2 occur in metabolic alkalosis due to:
eg: severe vomiting,hypokalemic states
– Decrease in CO2 are seen in: eg: renal failure
Calcium Ca++
• In body fluids mainly an extracellular cation —4.5-5.5mEq/L
• Most abundant in body but: 99% in teeth and bones
• Needed for nerve transmission, vitamin B12 absorption, muscle contraction & blood clotting
• Inverse relationship with Phosphorus
• Vitamin D needed for Calcium absorption
• The most abundant mineral in the human body
Food sources for calcium ions
Dairy foods: Milk, yogurt, cheese
Leafy green vegetables: Broccoli, kale, spinach
Fruits: Oranges
Beans and peas: Tofu, peanuts, peas, black beans, baked beans
Fish: Salmon, sardines
Miscellaneous: Sesame seeds, blackstrap molasses, corn tortillas, almonds, brown sugar
How it functions in body metabolism
• 99% of total body calcium is stored in the bones and teeth
• 1% is found throughout the body in blood, muscle, and the fluid between cells
45% circulates as free Ca ions
40% bound to albumin
15% bound to anions
• Ionized calcium is usually a more sensitive and specific for calcium disorders
Physiological function of calcium ions
• The normal transmission of nerve impulses
• Calcium flows into nerve cells and stimulates the release of molecules called neurotransmitters
• The role in muscle contraction
• Healthy blood pressure
• The initiation of blood clotting and
• The regulation of various hormones and enzymes
Physiological function of calcium ions
Can it be synthesized by the human body or must it come from other sources
• Body needs calcium to build and maintain strong bones and teeth
• Absorb calcium every day from dietary intake because body does not make calcium
Clinical Features: Hypocalcemia
• Serum Calicum < 4.3mEq/L
• Results from low intake, loop diuretics, parathyroid disorders, renal failure
• Treatment: diet/IV therapy
• What are some medical conditions that may cause hypocalcemia?
Hypoparathyroidism (low PTH levels = decreased release of Ca from bones)
S/P thryoid surgery (low Calcitonin = decreased release of Ca from bones)
Acute pancreatitis
Crohns Disease (inflammatory bowel disease)
Hyperphosphatemia (ESRF)
• What are some other conditions that might cause low Ca?
GI losses – nasogastric suctioning, vomiting, diarrhea
Long term immobilization
Lactose intolerance
• If hypocalcemia is prolonged, the body will utilize stored Ca from bones. What complication might arise?
Fractures (late sign)
Treatment of hypocalcaemia
Calcium, Vitamin D
Clinical Features: Hypercalcemia
• Serum Ca > 5.3mEq/L
• Results from hyperparathyroidism, some cancers, prolonged immobilization
• S/S muscle weakness, renal calculi, fatigue, altered LOC, decreased GI motility, cardiac changes
• Treatment: medication/ IV therapy
• What are some medical conditions that may cause hypercalcemia?
Hyperparathyroidism (high PTH levels = increased release of Ca from bones)
Paget ’s disease
Some Cancers – Multiple Myleoma
Chronic Alcoholism (with low serum phosphorus)
• What are some other conditions that might cause low Ca?
Excessive intake of Ca OR Vitamin D
Excessive intake of OTC antacids
If hypercalcemia is uncorrected, AV block and cardiac arrest may occur
Magnesium Mg 2+
• The eighth most abundant element in the earth’s crust
• Body concentration: 1.5-2.5mEq/L
• Most located within ICF
• Needed for activating enzymes, electrical activity, metabolism of proteins, DNA synthesis
• Regulated by intestinal absorption and kidney
• Magnesium plays important roles in the structure and the function of the human body
Food Sources for magnesium ion
• Green vegetables such as spinach because the center of the chlorophyll molecule (which gives green vegetables their color)
• Legumes (beans and peas)
• Nuts and seeds
• Unrefined grains
• Tap water (varies according to the water supply)
How it functions in body metabolism
• Activates more than 300 enzymes (energy-related)
• Regulates (Ca, K, and Na) transmission of nerve impulses. Calcium contract muscles and magnesium relax muscles
• Ensures proper DNA and RNA formation and function
• Facilitates PTH secretion
• Helps to regulate blood sugar levels, promotes normal blood pressure
• Involve in energy metabolism and protein synthesis
Metabolism of magnesium ion in the body
• Healthy individuals absorbs 40-60% magnesium consumed
• Absorption enhanced by calcium, phosphorus and fat
• Long term storage in the bones
• Kidney is the organ regulate magnesium homeostasis
• 90% of filtered magnesium is reabsorbed by the kidneys in response to salt and H2O reabsorption
Can it be synthesized by the human body or must it come from other sources
• Magnesium is a mineral, so therefore just like calcium, magnesium must be absorbed through dietary intake
• 50% of total body magnesium is found in bone
• The other half is found predominantly inside cells of body tissues and organs
• Only 1% of magnesium is found in blood, but the body works very hard to keep blood levels of magnesium constant
Clinical Features: Hypomagnesemia
• Serum < 1.5mEq/L
• Results from decreased intake, prolonged NPO status, chronic alcoholism & nasogastric suctioning
• S/S: muscle weakness, cardiac changes, mental changes, hyperactive reflexes & other hypocalcemia S/S
• Treatment: replacement IV therapy restore normal Ca levels (Mg mimics Ca) seizure precautions
Clinical Features: Hypermagnesemia
• Serum>2.5mEq/L
• Results from renal failure, increased intake
• S/S: Flushing, lethargy, cardiac changes (decreased HR), decreased respiratory, loss of deep tendon reflexes
• Treatment: Restrict intake,diuretic
Concentration for expressing electrolytes:
The concentration of electrolytes in solution is expressed in terms of milli equivalents (mEq)
• Refers to the chemical activity of an electrolyte
• Is related to the total number of ionic charges in solution and considers the valence (charge) of each ion
• For a given chemical compound, the milli equivalents of cations equals that of anions
Example: a solution of NaCl will contain the same number of milliequivalents of Na+ (the cation) as it will Cl- (the anion)
• There is a trend to shift from using mEq to using mg of the given ion
• Beware that this can be confusing! They are not EQUIVALENT!!! And mg of a given ion is not equivalent to mg of the compound
For example: mEq CaCl2 is not equal to mg CaCl2 which is not equal to mg Ca ion
Milli equivalents Formula:
• mEq = represents amount in milligrams, of a solute equal to 1/1000 of its gram equivalent weight taking into account the valence of the ions.
mEq = mg x valence / atomic, molecular or formula weight
mg = mEq x atomic, molecular or formula weight / valence
Equivalent weight = formula weight divided by the total valence
Equiv Weight (g) = atomic, molecular or formula weight / valence
Acids and Bases and Buffers
• Acids– Release H+ into solution
• Bases– Remove H+ from solution
• Acids and bases– Grouped as strong or weak
• Buffers: Resist changes in pH
– When H+ added, buffer removes
– When H+ removed, buffer replaces
• Types of buffer systems
– Carbonic acid/bicarbonate
– Protein
– Phosphate
Acid-base Balance
The importance of pH control
• The pH of the ECF remains between 7.35 and 7.45
– If plasma levels fall below 7.35 (acidemia), acidosis results
– If plasma levels rise above 7.45 (alkalemia), alkalosis results
– Alteration outside these boundaries affects all body systems
e.g. can result in coma, cardiac failure, and circulatory collapse
Regulation of blood pH
• The lungs and kidneys play important role in regulating blood pH
• The lungs regulate pH through retention or elimination of CO2 by changing the rate and volume of ventilation
• The kidneys regulate pH by excreting acid, primarily in the ammonium ion (NH4+), and by reclaiming HCO3- from the glomerular filtrate (and adding it back to the blood)
Mechanisms of pH control
• Buffer system consists of a weak acid and its anion
• Three major buffering systems:
– Carbonic acid-bicarbonate
• Buffers changes caused by organic and fixed acids
– Protein buffer system
• Amino acid
– Hemoglobin buffer system
• H+ are buffered by hemoglobin
Carbonic Acid-Bicarbonate Buffering System
• Carbonic acid-bicarbonate buffer system
– CO2 + H2O ßà H2CO3 ßà H+ + CO3–
• Has the following limitations:
– Cannot protect the ECF from pH changes due to increased or depressed CO2 levels
– Only functions when respiratory system and control centers are working normally
– It is limited by availability of bicarbonate ions (bicarbonate reserve)
Carbonate buffer system
H2CO3 + H2O ↔ H3O+ + HCO3–
• Excess acid (H3O+) in the body is neutralized by HCO3-
• H2CO3 + H2O ← H3O+ + HCO3–
Equilibrium shifts left
• Excess base (OH-) reacts with the carbonic acid (H2CO3)
• H2CO3 + OH- → H2O + HCO3-
Equilibrium shifts right
Protein buffer system
• Proteins contain – COO– groups, which, like acetate ions (CH3COO–), can act as proton acceptors.
• Proteins also contain – NH3+ groups, which, like ammonium ions (NH4+), can donate protons.
• If acid comes into blood, hydronium ions can be neutralized by the – COO– groups
– COO– + H3O+ → – COOH + H2O
• If base is added, it can be neutralized by the – NH3+ groups
– NH3+ + OH– → – NH2 + H2O
Phosphate buffer system
• The phosphate buffer system (HPO42-/H2PO4–) plays a role in plasma and erythrocytes.
H2PO4– + H2O ↔ H3O+ + HPO42-
• Any acid reacts with monohydrogen phosphate to form dihydrogen phosphate
H2PO4– + H2O ← HPO42- + H3O+
• The base is neutralized by dihydrogen phosphate
H2PO4– + OH– → HPO42- + H3O+
Kidney excretion of H+
– Metabolic reactions produce nonvolatile acids
– One way to eliminate this huge load is to excrete H+ in urine
– In the proximal convoluted tubule, Na+ /H+ antiporters secrete H+ as they reabsorb Na+
– Intercalated cells of collecting duct include proton pumps that secrete H+ into tubule fluid
– Urine can be up to 1000 times more acidic than blood
– 2 other buffers can combine with H+ in collecting duct
• HPO42- and NH3
Acid-base imbalances
– Normal pH range of arterial blood 7.35-7.45
• Acidosis – blood pH below 7.35
• Alkalosis – blood pH above 7.45
– Major physiological effect of
• Acidosis – depression of synaptic transmission in CNS
• Alkalosis – overexcitability of CNS and peripheral nerves
Metabolic acidosis/alkalosis
• Results from changes in HCO3– concentration
– Metabolic acidosis – abnormally low HCO3– in systemic arterial blood
• Loss of HCO3– from severe diarrhea or renal dysfunction
• Accumulation of an acid other than carbonic acid – ketosis
• Failure of kidneys to excrete H+ from metabolism of dietary proteins
• Hyperventilation can help
• Administer IV sodium bicarbonate and correct cause of acidosis
Clinical Features: Metabolic acidosis
– Abnormally high HCO3– in systemic arterial blood
• Nonrespiratory loss of acid – vomiting of acidic stomach contents, gastric suctioning
• Excessive intake of alkaline drugs (antacids)
• Use of certain diuretics
• Severe dehydration
• Hypoventilation can help
• Give fluid solutions to correct Cl–, K+ and other electrolyte deficiencies and correct cause of alkalosis
Monograph of Sodium chloride
Name: sodium chloride
Chemical formula: Nacl
Molecular weight: 58.4
Standards: Sodium Chloride contains not less than 99.0 percent and not more than 100.5 percent of NaCl, calculated on the dried basis
Synonyms: common salt
Method of Preparation:
Industrially, Laboratory
Industrially: By the evaporation of sea water
Laboratory: By neutralization process
Chemical reaction:
NaOH + HCl àNaCl + H2O
Properties of Sodium chloride:
Description:
White or colourless crystals or a white crystalline powder
Solubility: Freely soluble in water
Test for purity
•Appearance of solution
•Acidity or alkalinity
•Bromide
•Iron
•Arsenic
•Loss on drying
•Calcium and magnesium
•Sulphates
• Heavy metals
• Barium
•Iodide
Assay Principle
Precipitation titration, Modified Volhards Method
Nitrobenzene /dilute HNO3
NaCL + AgNO3 ————————————-à AgCl + NaNO3 + AgNO3
excess
NH4SCN + AgNO3 à AgSCN + NH4NO3
Unreacted
NH4SCN + Ferric alum à Ferric thiocyanate
Storage: Store protected from light
Medicinal uses:
•Manufacturing dialysis fluid
•Electrolytic replenisher
•Common Salt
Monograph of Potassium chloride
Name: Potassium chloride
Chemical formula: KCl
Molecular weight: 74.6
Standards: Potassium Chloride contains not less than 99.0 per cent and not more than 100.5 per cent of KCl, calculated on the dried basis
Synonyms: Sylvine, sylvite
Method of
Preparation:
It is prepared by neutralization of potassium hydroxide and hydrochloric acid
KOH + HCl à KCl + H2O
Properties of Potassium chloride:
Description: Colourless, Elongated, Prismatic or Cubical crystals or White granular powder, Odourless
Solubility: Freely soluble in water; insoluble in ethanol
Test for purity
•Iodides
•Appearance of solution
•Bromides
•Heavy metals
•Iron
•Arsenic
•Calcium and magnesium
•Sulphates
• Barium
•Loss on Drying
•Acidity or alkalinity
Assay Principle
Precipitation titration, Mohr’s Method
KCL + AgNO3 à AgCl + KNO3
Indicator: Potassium chromate
Colour change: Yellow to Brick red
Storage: Store protected from moisture
Medicinal uses:
•Seasoning agent
•Electrolytic replenisher
•Gelling agent
•Yeast food
Monograph of Calcium Gluconate
Name: Calcium Gluconate
Chemical formula: C12H22CaO14. H2O
Molecular weight: 448.4
Standards: Calcium gluconate contains not less than 98.0 per cent and not more than 102.0 per cent of calcium gluconate, calculated on the dried basis
Properties of Calcium gluconate :
Description: Colourless or White granular powder, Crystalline, Odourless, Stable in air
Solubility: Freely soluble in boiling water; insoluble in ethanol, chloroform and ether
Assay of calcium gluconate
Buffer: Ammonia and ammonium chloride solution
Indicator: Modarant Black II
Colour change: Pink – blue
Storage: Store protected from moisture
Medicinal uses:
•Hypocalcaemia
•Electrolytic replenisher
Oral Rehydration Salts
Name: Oral Rehydration Salts
Standards: Oral Rehydration Salts contain not less than 90.0 percent and not more than 110.0 percent of the stated amount of Dextrose (anhydrous) or Dextrose Monohydrate (as appropriate) and of the requisite amounts of sodium, Na, potassium, K, chloride, Cl, and citrate, C6H5O7, calculated from the stated amounts of the relevant constituents.
ORS Powder
Oral Rehydration Salts are dry, homogeneously mixed powders Containing Dextrose, Sodium Chloride, Potassium Chloride and either Sodium Bicarbonate or Sodium Citrate for use in oral rehydration therapy after being dissolved in the requisite amount of water
Storage:
•Store protected from moisture in sachets, preferably made of aluminium foil,
•containing sufficient powder for a single dose or for a day’s treatment or
•for use in hospitals, in bulk containers containing sufficient quantity to produce a volume of solution appropriate to the daily requirements of the hospital concerned
Labeling. The label states
(1) For sachets, the total weights, in g, of each constituent
(2) For bulk containers, the weights, in g, of each constituent in a stated quantity, in g, of the oral powder
(3)The molar concentration in millimoles per litre of sodium, potassium, chloride and citrate ions, and of dextrose as well as the total osmolar concentration in mOsmol per litre of the solution prepared from the oral powder
(4) The total weight of the contents of the container
(5) The directions for use
(6) That any portion of the solution prepared from the oral powder that remains unused for 24 hours after preparation should be discarded
(7) The storage conditions
Properties of ORS:
Description: white to creamy-white, amorphous or crystalline powder, odourless
Solubility: Freely soluble in water
Test for purity
•Uniformity of weight
•Seal test
•Other test as per oral powders
Medicinal uses:
•Maintaining acid base balance in body fluid
•Electrolytic replenisher
Summary
•The compositions of intracellular and extracellular fluids
•The movement of fluid that takes place within the ECF, between the ECF and the ICF, and between the ECF and the environment
•The hormones play important role in regulating fluid and electrolyte balance
Electrolytes play a major role in the body fluid compartments: Osmotic regulation, myocardial, acid base balance, blood coagulation
• The cations: Sodium, potassium, magnesium
• The anion: Chloride, phosphate, bicarbonate, sulphate
• Sodium: Major cat ion found in ECF
Function: Nerve impulses, sodium potassium pump.
Deficiency: Hyponatraemia – Sodium ions replacement
Hypernatraemia-Less sodium content
• Potassium: Higher concentration in the ICF
Maintains intracellular, functioning of excitable cells
Deficiency: Hypokalaemia -Replacement of potassium ions
Hyperkalaemia- Insulin + glucose, bicarbonate
• Chloride: The major extracellular anion, 97-107 mEq/L,regulated by ADH,maintains body metabolism, water and acid base balance, mainly with sodium ions
Deficiency: Hyperchloremia:Serum level > 106mEq/L
Treatment: restore fluid & electrolyte balance
Hypochloremia: Serum level > 106mEq/L
Treatment: restore fluid & electrolyte balance
• Phosphate PO4- : Plasma 1.7-2.6 mEq/liter, important acid-base buffer in body fluids
Deficiency:
Hypophosphatemia: Serum level < 1.8mEq/L
Treatment: Diet/ IV therapy
Hyperphosphatemia Serum level> 2.6mEq/L
Treatment: Diet; hypocalcemic interventions
Medications: phosphate binding
• Bicarbonate: Second most prevalent extracellular anion, 22-28mEq/L concentration, principle buffer of the body pH
• Change in the concentration of bicarbonate ions can form metabolic acidosis and metabolic alkalosis
• Calcium: Mainly an extracellular cation —4.5-5.5mEq/L,99% of total body calcium is stored in the bones and teeth,1% is found throughout the body in blood, muscle, and the fluid between cells.
Function: Nerve impulses, releases neurotransmitters, muscle contraction, blood clotting, regulation of blood clotting
Deficiency: Hypocalemia: Serum calcium < 4.3mEq/L,
Treatment: diet and IV therapy
Hypercalcemic: Serum Ca > 5.3mEq/L,
Treatment: Medication / IV therapy
• Magnesium: 1.5-2.5mEq/L, located within ICF
– Function: For activating enzymes, electrical activity, metabolism of proteins, DNA synthesis
– Deficiency : Hypomagnesemia: Serum < 1.5mEq/L
Treatment: Replacement/ IV therapy
Hypermagnesemia: Serum>2.5mEq/L
Treatment: Restrict intake, diuretic
• Milli equivalents: Represents amount in milligrams, of a solute equal to 1/1000 of its gram equivalent weight taking into account the valency of the ions
• Acids: Release H+ into solution
• Bases: Remove H+ from solution
• Buffers: Resist changes in pH
• Types of buffer systems
– Carbonic acid/bicarbonate
– Protein
– Phosphate
• Metabolic acidosis– abnormally low HCO3- in systemic arterial blood
• Metabolic alkalosis- Abnormally high HCO3- in systemic arterial blood
•Sodium chloride: Commonly known as common salt, is prepared by evaporation of sea water, assayed by modified Volhards method and medicinally used as electrolyte replenisher and in the preparation of isotonic solution
•Potassium chloride: Prepared by neutralising potassium hydroxide with Hydrochloric acid, assayed by Mohr’s method and medicinally used as electrolyte replenisher, gelling agent, adsorbent and yeast food
Calcium gluconate: Prepared by treating gluconic acid with calcium carbonate, used in the treatment of calcium deficiency
• ORS: Available in the form of sachets with different flavours, used for maintaining acid base balance
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf