Narcotic Analgesics (OPIOID ANALGESICS)

Intended Learning Outcomes
At the end of the lecture, student will be able to
• Write on narcotic analgesics
• Chart the history of narcotic analgesics
• Recall the opioid receptors
• Classify the narcotic analgesics
• Recognize the specific uses of the narcotic analgesics
• Discuss the side effects of narcotic analgesics
• Understand the SAR of Morphine
• Describe the peripheral and nuclear modifications done to the morphine ring and its effect on the activity
• Summarize about narcotic antagonists and their uses
NARCOTIC ANALGESICS
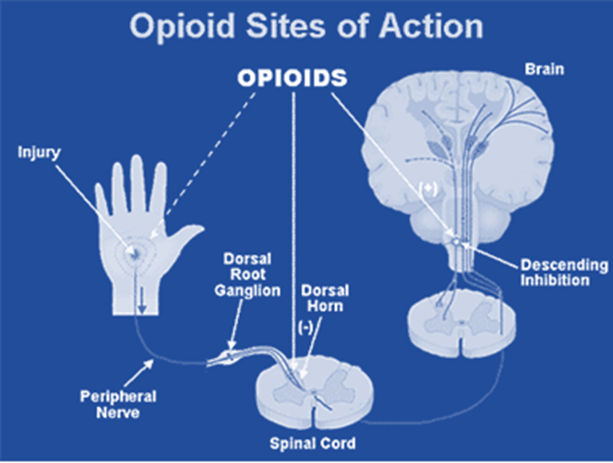
• Narcotic analgesics are drugs that relieve pain, can cause numbness and induce a state of unconsciousness.
• They work by binding to opioid receptor, which are present in the central and peripheral nervous system
• Narcosis: a state of stupor, drowsiness, or unconsciousness produced by drugs.
• Stupor: impaired consciousness with decreased reactivity to environmental stimulli
Opiate Analgesics
• Opium was known to man many centuries ago
• This is evident from the Ebers Papyrus and Homer’s Odyessey where the use of opium was mentioned
• Opium is obtained by making superficial incisions on the immature and unripe capsules of Papaver somniferum (or poppy plant)
• The exudate is air-dried and then powdered to give the official powdered opium
• A systematic study of the plant material led to the isolation and identification of the most important alkaloid known as morphine in 1803
• Other alkaloids isolated from opium include codeine, papaverine and thebaine
• The opium class of narcotic drugs are considered not only as the most potent and clinically useful agents causing depression of central nervous system, but also as very strong analgesics
• Morphine and morphine-like drugs are referred to as opioids or opiates. They are also known as narcotic analgesics
• ‘narcotic’ is derived from the Greek word ‘narcotic’ meaning drowsiness
• The term narcotic is now used to refer to dependence producing drugs
Historical perspective of Narcotic Analgesics
• First undisputed reference to “poppy juice” is found in the writings of Theophrastus in the third century B.C.
• The word opium being derived from the Greek word for “juice”
• The drug being obtained from the juice of the poppy Papaver sominiferum
• Arabian physicians were well versed in its uses and introduced the plant to the Orient
• Paracelsus, circa 1500, is credited with repopularising the drug in Europe, where it had fallen out of favor due to toxicity
• In the 18th century opium smoking became popular in the Orient and its ready availability in Europe led to considerable abuse
• Opium contains more than 20 alkaloids
• Morphine was first isolated by Friedrich Sertürner-1804
• He originally named it morphium after the Greek god of dreams, Morpheus.
• Isolation of other alkaloids soon followed, codeine in 1832 and papaverine in 1848
• By the middle of the 19th century, use of the pure alkaloids rather than crude opium was becoming widespread
• The problems of widespread addiction led to the search for a morphine antagonist, and in 1951 nalorphine was used in the RX of morphine overdose
• At the same time, the analgesic effects stimulated the development of a number of new drugs, including naloxone, pentazocine, butorphanol etc.
• By 1967, researchers had concluded that the complex interactions and differences between morphine and its derivatives could only be explained by the existence of more than one receptor type ® receptor dualism, Martin (1967)
• In 1973, following an approach developed by Goldstein, 3 groups of workers described saturable, stereospecific binding sites for opiate drugs
• Modifications of the morphine molecule are considered under the following headings:
• Early changes on morphine before Small, Eddy and their coworkers.
• Changes on morphine initiated in 1929 by Small, Eddy and coworkers.
• By- Eisleb and Schaumann in 1938 – discovery of potent analgesic meperidine (pethidine).
• By Grewe in 1946- synthesis of the morphinan group of analgesics.
Opioids
• Work by binding to opioid receptors, in the CNS, PNS and GIT
– Receptors mediate the beneficial, psychoactive effects and side effects
• Opiate – often used as a synonym for opioid- but the term opiate is limited to the natural alkaloids found in the resin of the opium poppy
• While opioid refers to both opiates and synthetic substances, as well as to opioid peptides
Opioid Receptors
Delta (δ) – Brain (pontine nuclei, amygdala, olfactory bulbs, deep cortex) & peripheral sensory neurons
Kappa (κ) – Brain (Hypothalamus), Spinal cord & peripheral sensory neurons
Mu (μ) – Brain (Cortex, thalamus), Spinal cord, intestinal tract & peripheral sensory neurons
Adverse effects of opioid
COMMOM SIDE EFFECTS
• Constipation
• Nausea
• Dizziness
• Head ache
• Dry mouth
SEVERE SIDE EFFECTS
• Respiratory depression
• Chest pain
• Abnormal heart beat
Classification of narcotic ligands
A) Narcotic agonists:
I. Natural Alkaloids of Opium
• Phenanthrenes: morphine, codeine, thebaine
• Benzylisoquinolines: papaverine, noscapine
II. Semi-synthetic Derivatives
• Diacetylmorphine (heroin), hydromorphone, oxymorphone hydrocodone, oxycodone
III. Synthetic Derivatives
• Phenylpiperidines: pethidine, fentanyl, alfentanyl, sufentnyl
• Benzmorphans: pentazocine, phenazocine, cyclazocine
• Propionanilides: methadone
• Morphinans: levorphanol
B) Mixed agonist-antagonist drugs (eg, nalbuphine, pentazocine) have agonist activity at some receptors and antagonist activity at other receptors; also included are the partial agonists (eg, butorphanol, buprenorphine).
C) Narcotic antagonists: Narcotic antagonists (eg, naloxone) do not have agonist activity at any of the opioid receptor sites. Antagonists block the opiate receptor, inhibit pharmacological activity of the agonist, and precipitate withdrawal in dependent patients.
The narcotic analgesics are usually classified on the basis of their basic chemical structures as discussed below along with a few classical examples from each category.
Morphine Sulphate BAN, Morphine Sulfate USAN.
• Obtained from unriped capsule of papaver somniferum
• Odourless, laevorotatory, white needle like crystals – bitter taste
• It is employed extensively as an analgesic, antitussive, adjunct to anaesthesia and nonspecific antidiarrheal agent.
• In small doses it helps to alleviate continued dull pain, whereas in large doses to relieve acute pain of traumatic or visceral origin
CODEINE BAN, USAN,
• Levorotatory, colourless & efflorescent crystals
• Not used as narcotic analgesic- has slight analgesic effect
• Used in the treatment of cough
• Codeine phosphate and Codeine Sulphate 10–20 mg/dose
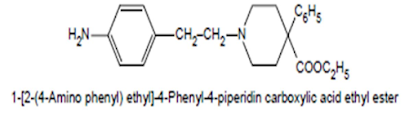
Anileridine Hydrochloride BAN, USAN, Anileridine INN
• It is a narcotic analgesic, having related chemical structure to that of pethidine
• Anileridine is more active than merperidine and has the same uses and limitations
• But with longer duration
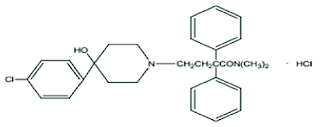
Lopramide:
• Chemically it is 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-N,N- dimethyl-2,2-diphenylbutanamide
• Loperamide is synthetic opioid that primarily affects opiate receptors in the intestine and is used to treat diarrheal
• One of the long-acting synthetic ANTIDIARRHEALS
Fentanyl Citrate BAN, USAN, Fentanyl INN,: Durogesic
• It is a white or almost white powder soluble in water, freely soluble in methanol, and sparingly soluble in alcohol.
• Fentanyl is related to pethidine and also to basic anilides with analgesic properties and is characterized by high potency, rapid onset, and short duration of action.
• A potent narcotic analgesic employed for the arrest of pain
• It may also be employed as an adjuvant for all such drugs mostly used for regional and general anaesthesia
Fentanyl Citrate
Methadone Hydrochloride BAN, USAN, Methadone INN,
• It is a Phenylpropylamine Analogues
• It is a potent narcotic analgesics having actions quantitatively comparable to morphine though slightly less potent than morphine as an analgesic. Besides, it exerts sedation and antitussive properties
• Similar actions to morphine-Longer duration of action
• Less problems with withdrawal.
– Can be used to wean heroin and morphine addicts off the drug.
– reduces withdrawal symptoms in people addicted to heroin
It is a white or almost white crystalline powder, freely soluble in ethanol and soluble in water.
Dextropropoxyphene Hydrochloride BAN, Propoxyphene Hydrochloride USAN, Dextropropoxyphene INN,
• Dextropropoxyphene is a narcotic analgesic possessing relatively milder actions and bearing structural resemblance to methadone.
• It is a white or almost white crystalline powder, very soluble in water and freely soluble in alcohol.
• It is usually used to control mild to moderate pain and chiefly
• used along with other analgesics having anti-inflammatory and antipyertic properties like paracetamol and aspirin.
Pentazocine INN, BAN, USAN
• It is a synthetic analgesic agent commonly used for the control of moderate to acute pain.
• It exerts some sedative actions.
• It causes incomplete reversal of the respiratory, cardiovascular and behavioural depression produced by either meperidine or morphine.
• It behaves both as an agonist and as an antagonist.
• It is reported to be 3 to 4 times less potent than morphine and about 50 times less potent than nalorphine.
• It is a white or almost white powder sparingly soluble in water, soluble in ethanol, and sparingly soluble in methylene chloride
Levorphanol Tartrate BAN, USAN, INN.
• It exists as white, odourless, crystalline powder, soluble in water and alcohol, insoluble in chloroform and ether.
• It is a potent narcotic analgesic having actions and structure similar to that of morphine.
• It is used effectively for the management of both moderate and
SAR of Morphine
SAR of Morphine was studied by
1. Modification of alicyclic ring
2. Modification of aromatic ring
3. Modification of 3o Nitrogen
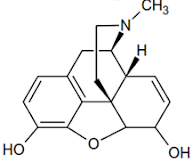
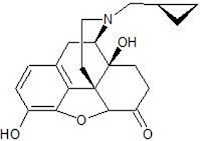
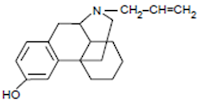
A. Morphine Structure and Chemistry
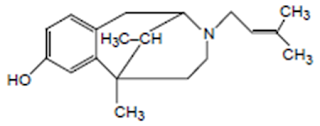
• The prototypic narcotic analgesic is (-)-morphine, the principal alkaloid obtained from the opium poppy (Papaver somniferum).
• Because of its natural source, morphine and morphine derivatives are referred to as opiates.
• Narcotic analgesics will be classified on the basis of their structural derivation from morphine
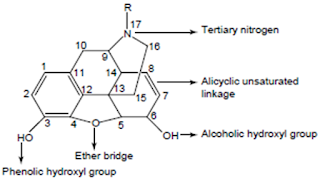
1. A rigid pentacyclic structure consisting of a benzene ring (A), two partially unsaturated cyclohexane rings (B and C), a piperidine ring (D) and a dihydrofuran ring (E).
Rings A, B and C are the phenanthrene ring system. This ring system has little conformational flexibility (the importance of this will be addressed later).
2. Two hydroxyl functional groups, a C3-phenolic OH (pKa 9.9) and a C6- allylic OH
3. An ether linkage between C4 and C5,
4. Unsaturation between C7 and C8,
5. A basic, 3o-amine function at position 17,
6. 5 centers of chirality (C5, C6, C9, C13 and C14) with morphine exhibiting a high degree of stereoselectivity of analgesic action. Only (-)-morphine is active!
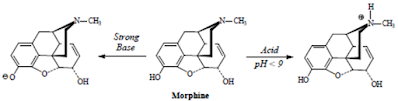
• Morphine contains both an acidic phenolic group and a basic tertiary amine functions.
• However,since the amine functions is significantly more basic than phenol group is acidic, thus, morphine as well as a majority of narcotic analgesics are functionally basic compounds both pharmaceutically (dosage forms) and physiologically.
• Hence, morphine exists as a cation at physiologic pH, and readily forms salts with appropriate acids (commercial products are sulfate and HCl):
Morphine Biodisposition/Metabolism
• Limited oral bioavailability due to extensive first pass metabolism (see below)
• Lipophilic, but less so than other opioids (i.e. heroin)
• Half-life: 2-3 hrs: Metabolism
• Elimination: Primarily in urine as the glucuronide
• Metabolism: 3- and 6-O-glucuronides, OND and O-methylation to codeine (minor)
III. “Peripherally Modified Morphines”: Ring A Analogues
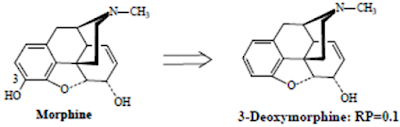
• Ring A and its 3-hydroxyl group is an important structural feature for analgesic activity
• Removal of the 3-OH group reduces analgesic activity 10-fold
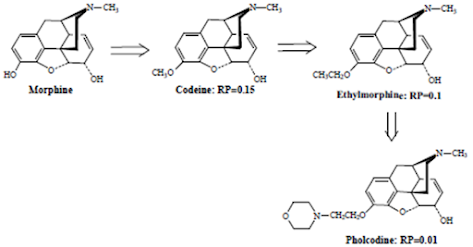
• Altering the C-3 OH by etherification as shown by the derivatives below reduces narcotic analgesic activity, the larger the ether group, the lower the analgesic activity.
• While less active as analgesics, compounds such as codeine possess very useful antitussive activity.
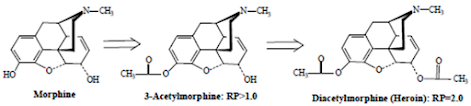
• Esterification (acetylation) of both the 3- and 6-OH groups yields heroin, a compound which is both more lipophilic and more potent
• The primary factor involved in increased analgesic potency is the increased lipophilicity and distribution to the CNS.
IV. “Peripherally Modified Morphines”: Ring C Analogues
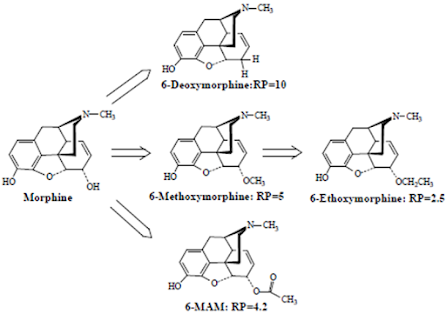
• The 6-OH of morphine are not required for analgesic activity as indicated by the relative potencies of the following morphine analogues.
• Elimination of the 6-OH actually enhances activity.
• Etherification of this group with relatively small alkyl group also increases activity.
• Esterification of the 6-OH as in the main hydrolysis metabolite of heroin, 6-MAM, also increases analgesic activity.
• This increased activity appears to result largely from the enhanced lipophilicity of these compounds and their increased ability to penetrate the CNS.
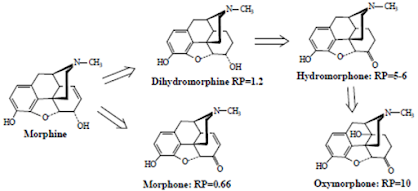
• The 7,8-double bond of morphine also is not required for analgesic activity as indicated by the relative analgesic potency of dihydromorphine.
• Also, oxidation of the 6-OH of dihydromorphine to yield hydromorphone further increases activity.
• And substitution of a 14-OH group on the hydromorphone structure as in oxymorphone produces a further increase in analgesic activity (RP= 10).
• Oxidation of the 6-OH of morphine directly as in morphone (without reduction of the 7,8-double bond) does not significantly alter analgesic activity.
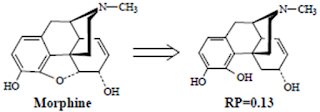
V. “Peripherally Modified Morphines”: Ring E Analogues
• Simple ring-opened analogues of morphine such as the compound below are less active.
• However, a number of morphine analogues in which this ring and its functionality are completed removed retain high analgesic activity.
VI. “Peripherally Modified Morphines”: Ring D Analogues and the Tertiary Amine Function
• Replacement of morphine’s N-methyl group by a hydrogen atom as in normorphine reduces analgesic activity to 1/8th that of morphine. Much of this decrease is due to increased polarity resulting in reduced BBB translocation to the CNS.
• The quaternary methiodide analogue of morphine is inactive when administered peripherally (by injection) but equi-active when administered directly into the CNS.
• The activity of this compound demonstrates the importance of cationic structure of morphine for morphine, and the role of structure in CNS penetration
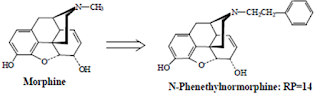
• Replacement of morphine’s N-methyl group with larger alkyl groups reduces activity.
• However, substitution with aralkyl groups significantly increases analgesic activity as illustrated by Nphenethylnormorphine
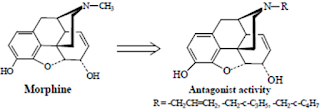
• Replacement of morphine’s N-methyl group with an allyl group (-CH2CH=CH2), amethylcyclopropyl group or a methylcyclobutyl group results in the emergence of opiate receptor antagonist activity
• Replacement of the potent narcotic agonist oxymorphone’s N-methyl group with an allyl group (-CH2-CH=CH2), a methylcyclopropyl group or a methylcyclobutyl group also results in the emergence of opiate receptor antagonist activity
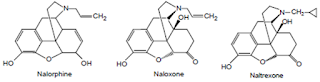
• Three of these compounds – naloxone HC1 (Narcan) naltrexone HCl (Trexan) and nalmefene (Revex) -are pure opiate antagonists (at mu, kappa and delta receptors) and
• are used for the reversal of the narcotic/respiratory depression induced by opiate agonists, as well as psychotomimetic actions.
• These compounds are also used as adjuncts to the maintenance of an opiate-free state in detoxified, formerly opiate-dependent patients.
VII. “Peripherally Modified Morphines”: The Thebaines
Annelation – adding a sixth ring across carbons 6 and 14 of the C ring of morphine – yields thebaine compounds such as etorphine which are extremely potent analgesics.
These compounds as typically used to immobilize large animals (elephants).
Nuclear modification
• At C2, if it is NH2, decreases NAA
• At C1, if it is halogens, decreases NAA
• Dihydromorphinone, at C14, if it is –OH, increases NAA
• Dihydrocodeinone, at C14, if it is –OH, increases NAA
• Instead of –OH, if it is -CH3 at C6 increases the activity
• If it is saturated, it has lesser activity, ex: synthetic morphine
Summarised SAR of Morphine
Narcotic antagonist
• An antagonist used to counteract the effects of narcotics (especially to counteract the depression of respiration)
• Narcotic antagonists: Prevents or abolishes excessive respiratory depression caused by the administration of morphine or related compounds.
• They act by competing for the same analgesic receptor sites.
• They are structurally related to morphine with the exception of the group attached to nitrogen
Classification of Narcotic antagonist
i. Pure antagonists (e.g. naloxone, naltrexone).
ii. Partial agonists of nalorphine type (e.g. Nalorphine, levallorphan, and cyclazocine).
iii. Partial agonists of morphine type (e.g. propiram, profadol).
Nalorphine Hydrochloride BAN, USAN, Nalorphine INN,
• It is a narcotic antagonist having certain agonist actions that reduce the depressant actions particularly of morphine together with other narcotic drugs
• Used as diagnostic agent to detect Morphine addiction
– It precipitates the withdrawal symptoms in patients addicted to Heroine & Methadone
• Decreases the drug dependence when administered along with morphine
• It has little antagonist effect towards barbiturate or general anaesthetic depression
• However, it has strong analgesic properties, but it is not acceptable for such use owing to the high incidence of undesirable psychotic effects.
• It is effectively employed to reverse narcotic-induced respiratory depression
Naloxone Hydrochloride BAN, USAN, Naloxone INN
• It is almost seven times more active than nalorphine in antagonizing the effects of morphine.
• It shows no withdrawal effects after long-term administration.
• It lacks not only the analgesic activity shown by other antagonists, but also all of the other agonist effects.
• At higher doses, Naloxone may be useful in the treatment of shock
Naloxone
• Pure opioid antagonist.
• μ, κ and δ-antagonist.
• No concomitant agonist properties.
• Naloxone- to counter the effects of opioid overdose, such as heroin or morphine in the life-threatening depression of the CNS, respiratory system, and hypotension.
Naltrexone
• Opioid receptor antagonist.
• μ, κ and δ-antagonist.
• Used in the management of alcohol and opioid dependence.
Levalorphine
• Levallorphan resembles nalorphine in its pharmacological action and is about five times more effective as a narcotic antagonist.
• It is useful in combination with analgesics, such as meperidine, alphaprodine, and levorphanol to prevent the respiratory depression usually associated with these drugs.
Summary
• Morphine was isolated in 1804
• Synthetic derivatives were prepared only a century later
• The opoid receptors are Mu, kappa and delta
• The narcotic analgesics are classified as
– Naturally occurring opium alkaloids: Morphine, Codeine
– Semi-synthetic opium derivatives:Heroin ,Oxymorphone
– Synthetic morphine substitutes: Pethidine,Levorphanol,
– Miscellaneous: Pentazocine
• Narcotic analgesics are used in chronic pain
• Most of the narcotic analgesics are habit forming
• Morphine is taken as reference to compare the SAR of modified substances. Its NAA (Narcotic analgesic activity) is considered as 100
• Periheral and nuclear modifications are done to the morphine ring and the activity is compared
• Narcotic antagonists used to counteract the effects of narcotics
– (especially to counteract the depression of respiration)
• Drugs used to counteract the effects of narcotics are
• Nalorphine hydrochloride
• Levallorphan tartarate
• Naloxone hydrochloride
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf