Nomenclature
of Optical Isomers
Session Objectives
By the end of this
session, students will be able to:
• Explain D & L system
• Explain the rules for assigning R & S Configuration
Enantiomers can be described as (+) or (-)
• The direction in which light is rotated is not dependent
on whether a stereogenic centre is R or S
• An (R) compound is equally as likely to be (+) as
(–)—of course, if it is (+) then its (S) enantiomer must be (–)
• The labels (+) and (–) were more useful before the days of
X-ray crystallography, when chemists did not know the actual configuration of
the molecules they studied, and
• Could distinguish two enantiomers only by the signs of
their specific rotations
• We have already seen that a (+) or (-) sign indicates the
optical activity of an enantiomer
• Optical activity does not tell us the actual configuration
of an enantiomer
• It only gives us the information whether an enantiomer
rotates the plane-polarized light clockwise or anti-clockwise
How to designate configuration of enantiomer?
• Two systems to designate configuration of enantiomers: D
and L system
• R and S system (also known as the Cahn–Ingold–Prelog
system)
D and L system
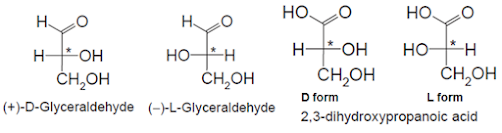
• Emil Fischer used glyceraldehyde as a standard for the D
and L system of designating configuration
• He arbitrarily took the (+)-glyceraldehyde enantiomer and
assigned this as D-glyceraldehyde
• Other enantiomer is the (-)-glyceraldehyde and this was
assigned as L-glyceraldehyde
• Only difference in the following structures, which is the
orientation of the hydroxyl group at the chiral center
• In the case of D-glyceraldehyde the –OH group on the
chiral carbon is in on the right hand side, whereas in L-glyceraldehyde it is
on the left
• No correlation between D and L configurations, and (+) and
(-) rotations
• It can be D(+) or D(-) and L(+) or L(-)
• D and L system is common in biology/biochemistry
especially with sugars and amino acids
• For example, D-glucose, L-rhamnose and L-alanine
Describing R & S Configuration
• Set of rules to assign a letter R or S, to describe the
configuration of groups at chiral center in the molecule
• R- From the Latin, rectus, straight, correct; to
show that the order of priority of groups on a chiral center is clockwise
• S- From the Latin, sinister, left; to show that the
order of priority of groups on a chiral center is counterclockwise
• For a given sample of a pure enantiomer, the absolute
configuration must be determined experimentally by X-ray analysis of a
derivative that has a chiral center with known configuration
• A system for designating the absolute configuration of a
chiral center was devised in the late 1950s by R. S. Cahn and C. K. Ingold in
England and V. Prelog in Switzerland and is named after them with CIP rules
• It has been incorporated in IUPAC rules of nomenclature
also
Priority rules
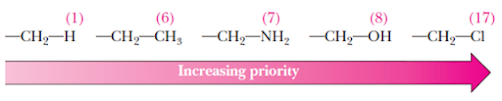
Rule 1: Each atom
bonded to the chiral center is assigned a priority based on atomic number; the
higher the atomic number, the higher the priority
Rule 2: If
priority cannot be assigned on the basis of the atoms bonded directly to the
chiral center look at the next set of atoms and continue until a priority can
be assigned.
Rule 3: Atoms
participating in a double or triple bond are considered to be bonded to an
equivalent number of similar “phantom” atoms by single bonds.
• That is, atoms of the double bond are duplicated, and
atoms of a triple bond are triplicated.
Note: Priority
assignment is made at the first point of difference between groups.
• A common mistake is to assume that larger groups must
always have higher priority, but this might not necessarily be the case. For
example, a –CH2Cl group has priority over a -CH2CH2CH2CH3
group because the Cl atom is the first point of difference
Rule 4: Having
decided on the priority of the four groups, one has to arrange (rotate) the
molecule in such a way that group 4, i.e. the lowest priority, is pointing away
from the viewer
• Then an arrow from group 1 to 2 to 3 is to be drawn. If
the direction is clockwise, it is called an (R)-isomer. If it is
anti-clockwise, it is called an (S)-isomer
• R & S enantiomers of 2,3-dihydropropanoic acid
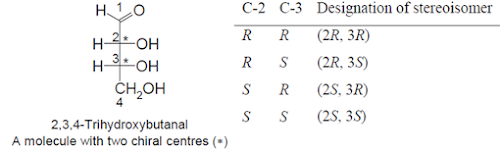
• When there is more than one stereocentre (chiral carbon)
present in a molecule, it is possible to have more than two stereoisomers.
• It is then necessary to designate all these stereoisomers
using the (R) and (S) system. In 2,3,4-trihydroxybutanal, there are two chiral
carbons at C-2 & C-3