Stereoselective & Stereospecific Reactions
Session Objectives
By the end of this
session, students will be able to:
• Define Stereospecific and Stereoselective reactions with
examples
• Explain Stereochemistry in biphenyls
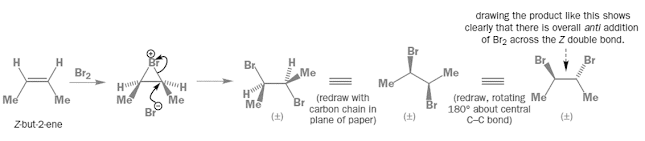
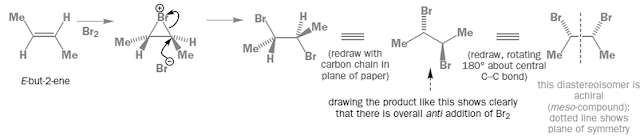
Stereospecific reactions
• A reaction is stereospecific provided the reactant can
exist as stereoisomers and each stereoisomeric reactant gives different
stereoisomeric product which may be (±) pair
• For example, bromination of alkenes is stereospecific
• Here geometry starting alkene decides which product
diastereoisomer is obtained
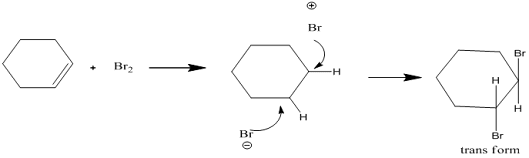
• A stereoselective reaction is one in which the reactant
not necessarily chiral but in which the reaction produces predominately or
exclusively one stereoisomeric form of the product
• For example, bromination cyclohexene, only one product of
stereoisomeric product trans-1,2-dibromocyclohexane is formed
• Here bromination proceeds through cyclic brominium, no
other chance of forming both isomers
• If its simple carbocation, both cis and trans would have
formed
Biphenyl compounds are optically active due to the
restricted rotation about the single bond
To exhibit optical activity
1) Both the rings shouldn’t have vertical plane of symmetry.
(I) is not resolvable and (II) is resolvable
Stereochemistry
in Biphenyls
• 2) Substituents in the ortho-positions must have a large
size, so that the steric effect prevents or restricts the rotation
• Should be co-axial (not coplanar) and non-superimposable
like (III) and (IV)
• Actual angle of inclination of two rings depends on nature
of substituent groups
• Usually rings are perpendicular to each other in vicinity
of 900
• Configuration (V) has shown plane of symmetry and (VI)
center of symmetry
• Theoretical and practical results have identified that
restricted rotation in biphenyl compounds is entirely due to spatial effects
• Rotation of phenyl groups depends on each other, various
positions corresponds to different conformations
• Because of steric hindrance, biphenyls have large energy
barriers separating two isomers of 75-105 KJ mol-1
• Such isomers are called atropisomers and condition is
called atropisomerism
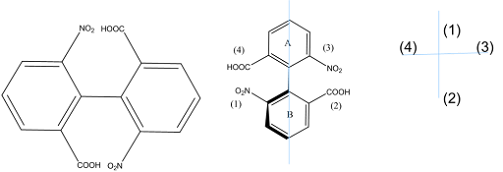
Absolute Configuration of Biphenyls
• Specification of absolute configuration is carried out as
follows:
• Turn the molecule so that ring A in the plane of paper is
at top while ring B is perpendicular to ring A at bottom
• Molecule is viewed from the bottom and assigned priorities
to ortho groups according to CIP rules
• Draw a cross and put the groups on the top of horizontal
line
• Put the groups of the bottom pair on the vertical line of
cross with the group in thick line front at the top, while the other at bottom
of vertical line
• If the sequence 1-2-3 clockwise shows R and anticlockwise
S configuration
• Its S configuration
Racemisation of biphenyl compounds
• As the optical activity of biphenyl compounds arises from
restricted rotation, it might be expected that racemization would not be
possible
• But optically active biphenyl compounds can be racemised
under suitable conditions like boiling in solution
• As per general theory, heating increases the amplitude of
vibrations of the two benzene rings and permit substituent groups slip
• In the planar configuration, energy content increases
because of steric repulsion
• Larger the groups, higher will be energy barrier and
slower the rate of racemization
• Order of steric hindrance produced by various groups are:
Br >> Me > Cl > NO2 > COOH >> OMe > F
• Based on the order of van der Waals radii of the groups
• Also nature and position of other susbstituent groups also
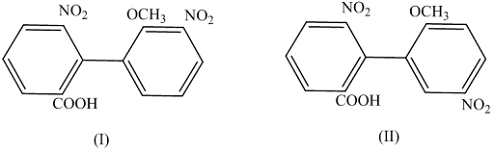
plays part in the rate of racemization
• For example, rate of racemization of (I) is much slower
than (II)
• Nitro group in position 3’ has a much greater stabilizing
effect than in 5’
• Order of stabilizing effect is NO2 > Br >
Cl > Me
Summary
• A reaction is stereospecific provided the reactant can
exist as stereoisomers and each stereoisomeric reactant gives different
stereoisomeric product which may be (±) pair
• Biphenyl compounds are optically active due to the
restricted rotation about the single bond
• Substituents in the ortho-positions must have a large
size, so that the steric effect prevents or restricts the rotation
• Because of steric hindrance, biphenyls have large energy
barriers separating two isomers of 75-105 KJ mol-1. Such isomers are called
atropisomers and condition is called atropisomerism
• Syn form is the one in which both the hydrogen atom and
hydroxyl group are on the same side
• When both are on opposite sides, configuration is anti