Adrenergic Blockers
Intended
learning outcomes
At the end of the
lecture the students will be able to
• Categorize adrenergic blockers
• State the specific uses for the various blockers
• Outline the synthesis of Tolazoline
Contents
• Categorize adrenergic blockers
• The specific uses for the various blockers
• The synthesis of Tolazoline
Adrenergic
blocking agents
• Adrenergic blockers are also called as antiadrenergic
drugs or sympatholytics
• Adrenergic blocking agents prevent the response of effector
organs to endogenous as well as exogenous adrenaline and noradrenaline
• These drugs block the actions of adrenergic drugs at alpha
(α) or beta (β) adrenergic receptors
• Useful in medicine, particularly in the treatment of
cardiovascular diseases
Mechanism
of Action of α -Adrenergic Blockers
• α -Adrenergic receptor response in clinical relevance
include α1 receptor mediated contraction of arterial and venous smooth muscle.
• α2 adrenergic receptors are involved in suppressing
sympathetic output, increasing vagal tone, facilitating platelet aggregation,
inhibiting the release of norepinephrine, and acetylcholine
• From nerve endings.
• Blockade of α1 receptors inhibits vasoconstriction induced
by endogenous catecholamines
• Vasodilatation may occur in both arteriolar resistance
vessels and veins.
• α 2 receptor regulates both central and peripheral
sympathetic neurons.
• Acceleration of presynaptic α2 receptors inhibits the
norepinephrine release.
• In some vascular beds, these drugs promote vasodilatation
through the release of nitric oxide (endothelial relaxing factor).
• Phenoxybenzamine inhibits the uptake of catecholamine from
the nerve terminals.
• Phentolamine and tolazoline are competitive α adrenergic antagonists
and block the receptor for 5-HT and it causes the release of histamine from the
mast cells, which is a potent vasodilator.
Mechanism
of Action of β-Adrenergic Receptor Blockers
• β adrenergic receptor antagonists slow the heart rate and
decrease the myocardial contractility, these prolongs the systolic conduction
and disturbs the ventricular fibres.
• Dimensions of the ventricle is decreased, oxygen
consumption is decreased, and thereby decreases the heart rate and aortic
pressure.
• In blood vessels, these drugs reduces the noradrenaline
release from the sympathetic terminals and decrease the renin from kidney due
to the blockade of β receptors
Classification:
Adrenergic blocking agents
I. Alpha receptor blocking agents:
a. Beta halo alkyl amines:
i. Dibenamine
ii. Phenoxy
benzamine
b. Natural and
dehydrogenated ergot alkaloids:
i. Ergotamine
ii. Ergocristine
iii. Ergocriptine
iv. Ergocornine
c. Imidazole
derivatives:
i.Tolazoline
ii.Phentolamine
d. Quinazolines:
i.Prazosin
ii.Terazosin
iii.Doxazosin
e. Miscellaneous
i.Indoramine
ii.Yohimbine
iii.Chlorpromazine
II.
Beta-receptor blocking agents
a. β-Blockers with
membrane stabilizing activity and intrinsic sympathomimetic property:
i.Oxprenalol
ii.Pindalol
b. Specifi c
β-blockers:
i.Timolol
ii.Nodalol
c. β-blockers with
membrane stabilizing activity
i.Propranolol
d. β-blockers with
cardio selective action
i. Acebutolol
ii. Atenolol
iii.Metaprolol
Tolazoline
Synonym: Priscoline
Chemical Structure
• Chemically it is 2-benzyl-4,5-dihydro-1H-imidazole
• Tolazoline is a member of the class of imidazoles that is
4,5-dihydro-1H-imidazole substituted by a benzyl group
Properties: It is
a white, bitter taste, crystalline compound with a slight aromatic odour,
soluble in water, alcohol, and chloroform, but sparingly soluble in ether.
Medicinal Uses:
• An alpha-adrenergic antagonist
• An antihypertensive agent and
• A vasodilator agent
Tolazoline Synthesis
It is prepared by condensation of an aminoether (obtained by
methanolysis of phenylacetonitrile) with ethylene diamine.
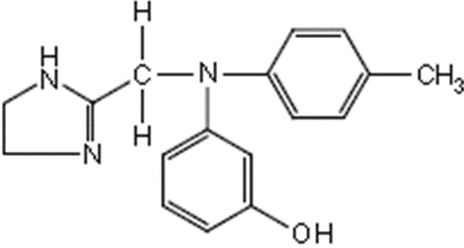
Phentolamine:
• Phentolamine is a synthetic imidazoline with alpha-
adrenergic antagonist activity
• Chemically it is
3-[N-(4,5-dihydro-1H-imidazol-2-ylmethyl)-4- methylanilino]phenol
Properties: It is
a white, odourless, bitter powder, soluble in water and alcohol
Medicinal Uses:
• A nonselective alpha-adrenergic antagonist
• It is used in the treatment of hypertension and
hypertensive emergencies
Phenoxybenzamine
(Dibenzyline)
• Phenoxybenzamine is a synthetic, dibenzamine alpha
adrenergic antagonist with antihypertensive and vasodilatory properties.
• Phenoxybenzamine non-selectively and irreversibly blocks
the postsynaptic alpha-adrenergic receptor in smooth muscle, thereby preventing
vasoconstriction, relieving vasospasms, and decreasing peripheral resistance.
• Reflex tachycardia may occur and may be enhanced by
blockade of alpha-2 receptors which enhances norepinephrine release.
• Phenoxybenzamine is reasonably anticipated to be a human carcinogen.
• Phenoxybenzamine is an aromatic amine.
Properties:
Colourless, crystalline compound soluble in alcohol, water, and chloroform.
Medicinal uses:
• A major use of phenoxybezamine is in the treatment of pheochromocytoma
(tumours of the adrenal medulla)
• It is used to treat peripheral vascular diseases, suchas
Raynaud’s syndrome
• It has also been used in the case of shock and frostbite
to improve blood flow to peripheral tissues
• Used in the treatment of shock and in the treatment of
pulmonary oedema
Prazosin:
(Minipress, Prazopress)
• It belongs to the class of drugs known as alpha-1 blockers
• Prazosin is an alpha-Adrenergic Blocker. The mechanism of
action of prazosin is as an Adrenergic alpha-Antagonist.
Properties: It is
a white crystalline powder, soluble in water and alcohol. A selective
α-antagonist,
Medicinal uses:
• prazosin, reduces peripheral vascular resistance and
lowers arterial blood pressure in both supine and erect patients.
• Used to treat hypertension of any degree.
• It has been used in decreasing cardiac overload
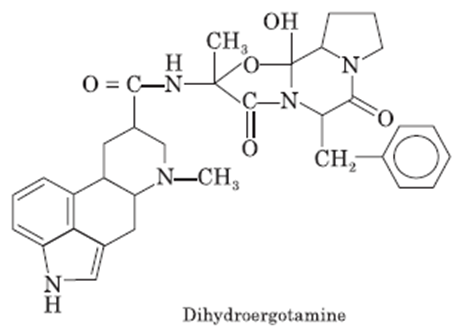
Dihydroergotamine
• Dihydroergotamine is an Ergotamine Derivative. The
chemical classification of dihydroergotamine is Ergotamines.
• Ergot alkaloids are widely used for therapy of acute
migraine headaches and include ergotamine and dihydroergotamine, both of which
act by causing vasoconstriction of the carotid artery beds.
• Ergot alkaloids have multiple side effects, but have
little effect on the liver and have not been clearly linked to instances of
clinically apparent acute liver injury.
• A 9,10alpha-dihydro derivative of ERGOTAMINE.
• It is used as a vasoconstrictor, specifically for the
therapy of MIGRAINE DISORDERS.
Methysergide
• An ergot derivative that is a congener of LYSERGIC ACID DIETHYLAMIDE
• It antagonizes the effects of serotonin in blood vessels
and gastrointestinal smooth muscle, but has few of the properties of other
ergot alkaloids
• Methysergide is used prophylactically in migraine and
other vascular headaches and to antagonize serotonin in the carcinoid syndrome
• Chemically it is
(6aR,9R)-N-[(2S)-1-hydroxybutan-2-yl]-4,7-dimethyl-
6,6a,8,9-tetrahydroindolo[4,3-fg]quinoline-9-carboxamide is an ergoline
alkaloid.