Local Anesthetics
Content
• Local Anesthetics
• Classification of local anesthetics
• Mechanism of Action
• Structure Activity Relationships of Local Anesthetics
• Various Local anesthetics drug profile
Local
Anesthetics
• Local anesthetics (LAs) are drugs which upon topical
application or local injection cause reversible loss of sensory perception,
especially of pain, in a restricted area of the body
• They block generation and conduction of nerve impulse at
• All parts of the neuron where they come in contact,
without causing any structural damage
Comparative features
of general and local anesthesia
Classification of
• Benzoic Acid
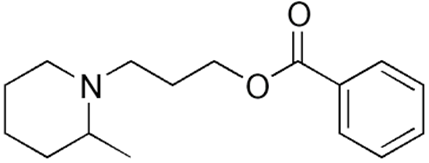
derivatives; Cocaine, Hexylcaine, Meprylcaine, Cyclomethycaine, Piperocaine
• Amino Benzoic acid
derivatives: Benzocaine, Butamben, Procaine, Butacaine, Propoxycaine,
Tetracaine, Benoxinate
• Lidocaine/Anilide
derivatives: Lignocaine, Mepivacaine, Prilocaine, Etidocaine.
• Miscellaneous:
Phenacaine, Diperodon, Dibucaine
• Injectable
anaesthetic
• Low potency, short duration
• Procaine
• Chloroprocaine
• Intermediate potency and duration
• Lidocaine (Lignocaine)
• Prilocaine
• High potency, long duration
• Tetracaine (Amethocaine)
• Bupivacaine
• Ropivacaine
• Dibucaine (Cinchocaine)
• Surface anaesthetic
• Soluble Insoluble
• Cocaine Benzocaine
• Lidocaine Butylaminobenzoate
• Tetracaine Oxethazaine
• Benoxinate
Mechanism
of Action of
• LAs block nerve conduction by decreasing the entry of Na+
ions during upstroke of action potential (AP).
• As the concentration of the LA is increased, the rate of
rise of AP and maximum depolarization decreases causing slowing of conduction Finally,
local depolarization fails to reach the threshold potential and conduction
block ensues
• The LAs interact with a receptor situated within the voltage
sensitive Na+ channel and raise the threshold of channel opening
• Na+ permeability fails to increase in response to an
impulse or stimulus
• Impulse conduction is interrupted when the Na+ channels
over a critical length of the fiber are blocked
A model of the axonal Na+channel depicting the site and mechanism of
action of local anaesthetics.
• The Na+ channel has an activation gate (‘m’ gate) near its
extracellular mouth and an inactivation gate (‘h’ gate) at the intracellular
mouth.
• In the resting state the activation gate is closed
• Threshold depolarization of the membrane opens the
activation gate allowing Na+ ions to flow in along the concentration gradient
within a few msec, the inactivation gate closes and ion flow ceases
• The channel recovers to the resting state in a
time-dependent Manner
• The local anaesthetic (LA) receptor is located within the
channel in its intracellular half.
• The LA traverses the membrane in its unionized lipophilic
from (B), reionizes in the axoplasm and approaches the LA receptor through the
intracellular mouth of the channel
• It is the cationic form (BH+) of the LA which primarily
binds to the Receptor
• The receptor has higher affinity or is more accessible to
the LA in the activated state compared to the resting state.
• Binding of LA to its receptor stabilizes the channel in
the inactive state and thus reduces the probability of channel opening
Structure Activity Relationships of Local Anesthetic
• The structure of most local anesthetic agents consists of three
parts
(a) A lipophilic ring that may be substituted
(b) A linker of various lengths that usually contains either
an ester or an amide
(c) An amine group that is usually a tertiary amine
The Aromatic Ring
• The aromatic ring adds lipophilicity to the anesthetic and
helps the molecule penetrate through biological membranes
• Substituents on the aromatic ring may increase the
lipophilic nature of the aromatic ring
• An SAR study of para substituted ester type local
anesthetics showed that lipophilic substituents and electron-donating
• Substituents in the para position increased anesthetic
activity
• The lipophilic substituents are thought to both increase
the ability of the molecule to penetrate the nerve membrane and increase their affinity
at the receptor site
• Electron-donating groups on the aromatic ring created a
resonance effect between the carbonyl group and the ring, resulting in the shift
of electrons from the ring to the carbonyl oxygen
• As the electronic cloud around the oxygen increased, so
did the affinity of the molecule with the receptor
• When the aromatic ring was substituted with an electron
withdrawing group, the electron cloud around the carbonyl oxygen decreased and
the anesthetic activity decreased
The Linker
• The linker is usually an ester or an amide group along
with a hydrophobic chain of various lengths
• When the number of carbon atoms in the linker is
increased, the lipid solubility, protein binding, duration of action, and
toxicity increases
• Esters and amides are bioisosteres having similar sizes,
shapes, and electronic structures
• The similarity in their structures means that esters and
amides have similar binding properties and usually differ only in their
stability in vivo and in vitro
• For molecules that only differ at the linker functional
groups, amides are more stable than esters and thus have longer half-lives than
esters
• Plasma protein binding may be more prevalent for the amide
anesthetics as well, contributing to the increased half-life
• The nature of the substituents on the aromatic ring can
affect the electronic nature of the linker and can contribute to the drug’s
potency and stability
• Substituents on the aromatic ring may also confer a steric
block to protect the linker from metabolism.
• The binding affinity and stability of the anesthetic
molecule is affected by the linker as well as the functional groups on the
aromatic ring
• Ester groups are more susceptible to hydrolysis than amide
functional groups because of the prevalence of esterases in the blood and the
liver
• The para-aminobenzoic acid (PABA) metabolite, common to
the ester class of drugs, is believed to be responsible for the allergic
reactions some patients have experience with local anesthetics
The Nitrogen
• Most local anesthetics contain a tertiary nitrogen
• Quaternary anesthetics applied to the external side of the
nerve membrane do not penetrate and cannot access the local anesthetic binding
site
Local
Actions
• The clinically used LAs have no/minimal local irritant
action and block sensory nerve endings, nerve trunks, neuromuscular junction, ganglionic
synapse and receptors (non-selectively)
• Structures which function through increased Na+
permeability
• They also reduce release of acetylcholine from motor nerve
endings Injected around a mixed nerve they cause anesthesia of skin and paralysis
of the voluntary muscle supplied by that nerve
Cocaine
methyl (1R,2R,3S,5S)-3- (benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]
octane-2-carboxylate
• A stimulant drug obtained from the leaves of two Coca
species native to South America, Erythroxylum coca and Erythroxylum
novogranatense
• Cocaine is the only local anesthetic with vasoconstrictive
properties. This is a result of its blockade of norepinephrine reuptake in the
autonomic nervous system.
• It is applied to certain areas of the body (for example,
the nose, mouth, or throat) to cause loss of feeling or numbness.
Hexylcaine
• Hexylcaine is a local ester-class anesthetic.
• Hexylcaine hydrochloride, also called cyclaine (Merck) or
osmocaine, is a short-acting local anesthetic.
• It acts by inhibiting sodium channel conduction.
Meprylcaine
• Meprylcaine (also known as Epirocaine and Oracaine) is a
local anesthetic with stimulant properties that is structurally related to
dimethocaine.
• Meprylcaine has a relatively potent inhibitory action on
the monoamine transporter and inhibits the reuptake of dopamine, norepinephrine
and serotonin.
Cyclomethycaine
• Cyclomethycaine is a local anesthetic.
• Used for pain on damaged skin,mucous membrane of rectum
and urinary bladder
Piperocaine
• Piperocaine is a local anesthetic drug developed in the
1920s
• Used as its hydrochloride salt for infiltration and nerve
blocks.
• In eye lotion
Benzocaine
• It is not water soluble but is ideal for topical
applications.
• The onset of action is within 30 seconds and the duration
of drug action is 10 to 15 minutes
• Benzocaine is used for endoscopy, bronchoscopy, and
topical Anesthesia
• Benzocaine is available as a 20% solution topical spray,
in a 1% gel for mucous membrane application
• 14% glycerin suspension for topical use in the outer ear
• Toxicity to benzocaine can occur when the topical dose
exceeds 200 to 300 mg resulting in methemoglobinemia
• can be directly applied to wounds and ulcerated surfaces
and remain in contact with the affected skin or mucous membrane
Butamben
• It is more efficacious than benzoate
• Butamben is anesthesia of mucus membranes other than the
eyes.
• Butamben is a lipophilic local anesthetic of the ester
class,
• Produces a differential nerve block of long duration,
• Used to provide prolonged analgesia for patients with
terminal malignancy.
Butacaine
• Local anaesthetics which are derivatives of p-aminobenzoic
acid
• Veterinary use
Propoxycaine
• Used in 1950s
• Combined with procaine to accelerate its onset of action
and provide longer lasting anaesthetic effect
Tetracaine
• Topical use
• To reduce pain or discomfort caused by mild skin
irritations such as sunburn or minor rashes
Benoxinate
• Enoxinate or BNX, is an ester-type local anesthetic,
• Which is used especially in ophthalmology and
otolaryngology.