Anti-neoplastic Agents

Learning Objectives
At the end of this lecture, student will be able to
• Describe various stages of cell cycle
• Explain Drug Resistance to Chemotherapy
• Classify antineoplastic agents
• Discuss the mode of action of Alkylating agents
• Illustrate uses and side effects of Alkylating agents
• Discuss the different antimetabolites as anticancer drugs
• Explain mechanism of action of antimetabolites
• Discuss the structures, uses of vinca alkaloids
• Describe the mode of action of compounds like cisplatin, asparaginase
• Explain the mode of action of Hormones for Antineoplastic therapy
• Discuss aspects involved in Immunotherapy
• Discuss the different plant products as anticancer drugs
• Explain mechanism of action of different plant products
• Discuss the different antibiotics as anticancer drugs
• Discuss miscellaneous agents as anticancer drugs
Antineoplastic Agents
• Antineoplastic agents are the drugs used for the treatment of cancer
• Cancer or neoplasm refers not to a single disease but to a group of diseases probably caused by several agents such as certain chemical compounds, radiant energy, certain viruses and cellular mutation of unknown origin
• The basic differences between cancer cells and normal cells are uncontrolled cell proliferation, decreased cellular differentiation, ability to invade surrounding tissue and ability to establish new growth at ectopic sites (metastasis)
• Cancer is more difficult to cure than bacterial infections as
• There are qualitative differences between human and bacterial cells, but the differences between normal and neoplastic human cells are mostly quantitative.
• Immune mechanisms and other host defenses are very important in killing bacteria and other foreign cells, whereas they play negligible role in killing cancer cells.
• A single cancer cell can reestablish the tumor, and it is extremely difficult to effect complete kill of cancer cells
• A higher proportion of resistant cells would be present, which would mean that retreatment with the same agent would achieve a lesser response than before
Cell cycle: –
• Cell cycle is divided into ‘s’ phase, where DNA replication takes place and ‘M’ phase where the mitosis takes place
• Between these two phases is G1 and G2, which are called as resting phases
• G1 is highly variable because of another phase G0
Typical duration:
• S = 10 – 20 hours
• G2 = 2 – 10 hours
• M = 0.5 – 1 hour
• G1 = highly variable
• Cells in the S or M phase are highly susceptible to anti-neoplastic agents unlike cells in resting phase which are resistant
• Most anticancer drugs block the biosynthesis or transcription of nucleic acids or they prevent cell division by interfering with mitotic spindles.
Cell-kill hypothesis:
• This hypothesis states that the effect of antitumor drugs on tumor cell populations follow first-order kinetics. This means that the number of cells killed is proportional to the dose.
• Thus, chemotherapy follows an exponential or log-kill model in which a constant proportion, not a constant number of cancer cells are killed.
• So, theoretically cancer chemotherapy can never reduce tumor populations to zero complete eradication requires another effect, such as the immune response.
Drug resistance
• Drug resistance to chemotherapy usually involves the selection of certain cell populations
• Populations of drug – resistance cells can be produced by
• Clonal evolution or by Mutation
• Mutagenic agents increase the frequency of generation of drug-resistant cells
• Many anti-tumor agents are mutagenic.
• Intracellular effects that cause drug resistance may be secondary to cellular adaptation or altered enzyme levels or properties
• For example, resistance to methotrexate involves increased levels of the target enzymes, “dihydrofolate reductase”.
Other modes of resistance to antimetabolites include
• Reduced drug transport into the cells
• Reduced affinity of the molecular target
• Stimulation of alternate biosynthetic pathways
• Impaired activation or increased metabolism of the drug
• A major factor in resistance to alkylating agents is the ability of tumor cell to repair DNA lesions such as cross-linkings and breakage of DNA strands caused by alkylation
• Chemotherapy is not the initial treatment used against cancer.
• If the cancer is well defined and accessible, surgery is preferred skin cancers and certain localized tumors are treated by radiotherapy.
• Generally, chemotherapy is important when the tumor is inoperable or has metastasized.
• Chemotherapy is finding increasing use as an ‘adjuvant’ after surgery to ensure that few cells remain to regenerate the parent tumors are killed.
General classification
Antineoplastic agents are classified as
• Alkylating agents
• Antimetabolites
• Antibiotics
• Plant products
• Hormones
• Miscellaneous agents
• Radioactive isotopes
• Immunotherapy
Alkylating agents
• Nitrogen mustards
– Mechlorethamine
– Cyclophosphamide
– Melphalan
• Alkyl sulphonate ester
– Busulfan
• Nitrosourea
• Lomustine
• Carmustine
• Aziridine
– Thiotepa
Antimetabolites
• Folate antagonist
• Methotrexate
• Purine antagonists
– 6-mercapto purine
– 6-thioguanine
• Pyrimidine antagonists
– 5-fluorouracil
– Cytarabine
Plant products
• Etoposide
• Taxol
• Camphothecin
• Vinblastine
• Vincristine
Antibiotics
– Dactinomycin
– Bleomycin
– Mitomycin
Hormones
– Dromostanolone
– Megestrol
Miscellaneous
– Cisplatin, Asparginase, Hydroxyurea
Immunotherapy
– Interferon α2a and 2b
Alkylating agents: (Present working hypothesis)
• Alkylation is defined as the replacement of hydrogen on an atom by an alkyl group. The alkylation of nucleic acids or proteins involves a substitution reaction in which a nucleophilic atom (nu) of the biopolymer displaces a leaving group from the alkylating agent.
Nu-H + alkyl-y → alkyl-nu + H+ + Y–
• The reaction rate depends on the nucleophilicity of the atom (S, N, O), which is greatly enhanced if the nucleophile is ionized.
• Alkylating agents produce cytotoxic, mutagenic and carcinogenic effects by reacting with cellular DNA.
• They also react with RNA and proteins. The most active clinical alkylating agents are bi-functional compounds capable of crosslinking DNA. The cross-linking process can be inter-strand or intra-strand.
• Inter-strand cross-linking occurs with mechlorethamine or other ‘two-armed’ mustards.
• Intra-strand cross-linking occurs with busulfan.
• In DNA, ‘7’position (Nitrogen) of guanine – ribose linkage susceptible to cleavage. This cleavage results in the deletion of guanine and also cleavage of 8-9 bond in the purine nucleus.
• Other base positions of DNA attacked by alkylating agents are N-2 and N-3 of guanine; N-3, N-1 & N-7 of adenine; O-6 of thymine; and N-3 of cytosine.
Meclorethamine
Use: – in the treatment of Hodgkin’s disease, lymphomas and mycosis fungoides can be treated with mechloethamine
Cyclophosphamide
Advantages
• Active orally and parenterally and can be given in fractionated doses over prolonged periods
Uses
• Active against multiple myeloma, chronic lymphocytic leukemia (CLL) and acute leukemia of children
• In combination with other C.T agents, it cures Burkett’s lymphoma and acute lymphoblastic leukemia (ALL) in children.
Toxic effects
• Alopecia, nausea and vomiting, leukopenia, sterile hemorrhagic cystitis.
Antimetabolites
• Antimetabolites are compounds that prevent the biosynthesis or use of normal cellular metabolites
• All the clinical agents are related to metabolites and cofactors in the biosynthesis of nucleic acids
• They are closely related in structure to the metabolite that is antagonized
• Most antimetabolites are effective cancer chemotherapeutic agents via interaction with the biosynthesis of nucleic acids
• Therefore several of the useful drugs used in antimetabolite therapy are purines, pyrimidines, folates, and related compounds
• Many antimetabolites are enzyme inhibitors, by combining with the active site as if they were the substrate or cofactor or bind to an allosteric regulatory site
• Most of these targeted enzymes and processes are involved in the regulatory steps of cell division and cell/tissue growth
• Sometimes, the antimetabolites must be transformed biosynthetically into the active inhibitor
• The administration of many purine and pyrimidine antimetabolites requires the formation of the nucleoside and finally the corresponding nucleotide for antimetabolite activity
• An antimetabolite and its transformation products may inhibit several different enzymes involved in tissue growth
• These substances are generally cell cycle specific with activity in the S phase
• Folate antagonist
• Methotrexate
• Purine antagonists
– 6-mercapto purine
– 6-thioguanine
• Pyrimidine antagonists
– 5-fluorouracil
– Cytarabine
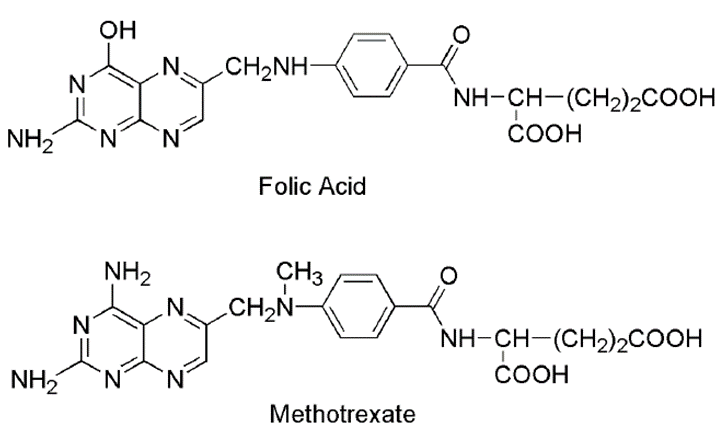
Folic acid Antagonist
Methotrexate
• Methotrexate is the classic antimetabolite of folic acid structurally derived by N-methylation of the para-aminobenzoic acid residue (PABA) and replacement of a pteridine hydroxyl by the bioisosteric amino group
• Methotrexate due to its structural similarity with folic acid inhibits the enzyme dihydrofolate reductase and there by prevent the formation dihydrofolic acid and tetrahydrofolic acid – which are needed for nucleic acid synthesis in tumor cell
• This drug competitively inhibits the binding of the substrate folic acid to the enzyme DHFR, resulting in reductions in the synthesis of nucleic acid bases, perhaps most importantly, and the conversion of uridylate to thymidylate as catalyzed by thymidylate synthetase
Use:
• In the treatment of acute lymphocytic leukemia and acute lymphoblastic leukemia.
• Since it has some ability to enter the CNS, it is used in the treatment and prophylaxis of meningeal leukemia.
Purine Antagonists
6-mercaptopurine
Use:
• In the treatment of acute lymphocytic leukemia and acute lymphoblasticleukemia.
• Since it has some ability to enter the CNS, it is used in the treatment and prophylaxis of meningeal leukemia
• 6-mercaptopurine is converted into the corresponding ribonucleotide, which is potent inhibitor of the conversion of 5phosphoribosyl pyrophosphate into 5-phosphoribosylamine, which is the rate controlling step in the synthesis of purines in microbial cells
• Mercaptopurine is used alone or with other chemotherapy drugs to treat acute lymphocytic leukemia
Toxicity: bone marrow depression and orogastrointestinal damage
6-thioguanine
• Thioguanine is converted into its ribonucleotide by the same enzyme that acts on 6-mercaptopurine. It is converted further into the di-& triphosphates. These species inhibit most of the same enzymes that are inhibited by 6-mercaptopurine.
• Thioguanine is also incorporated into RNA and its 2’-deoxy metabolite’ is incorporated into DNA
• Use: exclusively for the treatment of leukemias.
• Toxicity: bone marrow depression and orogastrointestinal damage
5-Fluorouracil
• In certain tumors uracil is used as the major precursor for the nucleic acid pyrimidine biosynthesis.
• 5-fluorouracil is the antimetabolite of uracil.
• 5-fluorouracil−−−−−−−−−−→5-fluoro-2-deoxyuridylic acid−−−−−−−−−−−−−−−−−−−−−−−−−→Thymidylate synthetase (inhibits –powerful competitive inhibitors)
• (Thymidylate synthetase – responsible for the conversion of 2deoxyuridylic acid to thymidylic acid – to pyrimidine synthesis in tumor cells)
• The tetrahydrofuranyl derivative of 5-fluorouracil (TEGAFUR) is active in clinical cancer and less myelosuppressant than 5-fluorouracil.
• TEGAFUR is slowly metabolized to 5-fluorouracil and so it is considered as a pro-drug
• Use: – in the treatment of carcinomas of breast, colon, pancreas, stomach and rectum
• Topically in the treatment of premalignant keratosis of skin
Floxuridine
• Floxuridine is a fluorinated pyrimidine monophosphate analogue of 5-fluoro-2′-deoxyuridine-5′-phosphate (FUDR-MP) with antineoplastic activity.
• As an antimetabolite, floxuridine inhibits thymidylate synthase, resulting in disruption of DNA synthesis and cytotoxicity
• This agent is also metabolized to fluorouracil and other metabolites that can be incorporated into RNA and inhibit the utilization of preformed uracil in RNA synthesis
• Floxuridine (FUDR) is a pyrimidine analogue used as an antineoplastic agent
• Used to treat hepatic metastases of gastrointestinal adenocarcinomas and for palliation in malignant neoplasms of the liver and gastrointestinal tract
Cytarabine
• Cytarabine is used in the treatment of acute myelogenous leukemia and CML
• This drug is a deoxycytidine analog
• It is active following intracellular activation to the nucleotide metabolite ara-CTP.
• The resulting ara-CTP is incorporated into DNA resulting in chain termination and inhibition of DNA synthesis and function
• Resistance can occur because of decreased activation or transport and increased catabolic breakdown
• Metabolic breakdown within the GI tract leads to poor bioavailability
• Toxicities include myelosuppression, leukopenia and thrombocytopenia, nausea and vomiting anorexia, diarrhea, and mucositis
Azathioprine
• Azathioprine is in a class of medications called immunosuppressants
• Azathioprine for the treatment of autoimmune disorders is significantly associated with an increased risk for developing acute myeloid leukemia (AML)
Antibiotics
Dactinomycin
Bleomycin
• Bleomycin is a glycopeptide antibiotic complex isolated from Streptomyces verticillus
Mitomycin
Daunorubicin
• Daunorubicin is used to treat leukemia and other cancers. It belongs to a class of drugs known as anthracyclines and works by slowing or stopping the growth of cancer cells.
Doxorubicin
• Doxorubicin is a type of chemotherapy drug called an anthracycline.
It slows or stops the growth of cancer cells by blocking an enzyme called topo isomerase II
• Acute lymphoblastic leukemia (ALL)
• Acute myeloblastic leukemia (AML)
• Bone sarcoma
• Breast cancer
Plant products
• Podophyllotoxin, obtained from the resin of the May apple, podophyllum peltatum has antineoplastic activity but is highly toxic.
• This lignin inhibits mitosis by destroying the structural organization of the mitotic apparatus. The newer analogues of podophyllotoxin eg., Etoposide are much better antineoplastic agents. This drug will inhibit topoisomerase II unlike podophyllotoxin
Etoposide
Use:
• In combination with other chemotherapeutic agents
• It is the first choice treatment for small cell lung cancer
• In refractory testicular tumors, acute non-lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphomas and Kaposi’s sarcoma.
Toxicity: –bone marrow suppression, alopecia, nausea and vomiting.
Vinca Alkaloids
• Are a family of important antitumor agents from plants.
• Isolated from periwinkle ‘Catharanthus rosea’.
• Composed of indole – containing moiety, Vindoline.
• Four closely related compounds have anti-tumor activity: Vincristine, Vinblastine, Vinrosidine, Vinleurosine.
• Among this Vincristine and Vinblastine are proved clinical agents.
Mode of Action:
• Vinca alkaloids cause mitotic arrest by promoting the dissolution of microtubules in cells.
• Microtubule crystals containing the alkaloids are formed in the cytoplasm.
• Vinblastine is the most active compound whereas Vincristine is the only compound to cause irreversible inhibition of mitosis.
Vincristine
Effective against acute leukemia
In the treatment of Hodgkin’s disease (combination chemotherapy)
Other tumors that respond to vincristine in combination with other chemotherapeutic agents include lympho-sarcoma, reticulum cell sarcoma, rhabdomyosarcoma, neuroblastoma and Wilms’ tumor.
Toxicity: –
Neurological (loss of deep tendon reflexes, pain and muscle weakness)
Vinblastine
• Used for the palliative of a variety of neoplastic diseases.
• One of the most effective single agents against Hodgkin’s disease
• In the treatment of advanced testicular germinal cell tumors
• In lymphocytic lymphoma, histiocytic lymphoma, mycosis fungoides, Koposi’s sarcoma, Leterer-Siwe disease, resistant choriocarcinoma and carcinoma of breast
Toxicity:
• Leukopenia
• Gastrointestinal and neurological symptoms
• Cellulitis and phlebitis.
Taxol
Camptothecin
Miscellaneous compounds
Cisplatin
Mode of action: –
• It interacts with DNA and Intra-strand crosslinks are produced and causes changes in DNA conformation that affect replication.
Use: –
• Used (in combination with bleomycin and vinblastin) for metastatic testicular tumors
• For the remission of metastatic ovarian tumors (a single agent or in combination)
• Other uses: in penile cancer, bladder cancer, cervical cancer, head & neck cancer and small cell cancer of the lung.
Toxicity: –
• Cumulative renal insufficiency associated with renal tubular damage
• Myelo suppression, nausea & vomiting and ototoxicity
Asparaginase
• Enzyme isolated commercially from E. Coli and E. Caratovora
• Asparagine is required for the biosynthesis of proteins.
• Although normal cells can synthesise ‘asparagine’ tumors such as acute lymphoblastic leukemia (ALL) lack this ability and depend on exogenous compound. • Administration of Asparaginase reduces the concentration
of Asparagine in plasma, making it unavailable to the leukemia cells.
Use: –
• To induce remissions in ALL and non-Hodgkin’s lymphoma.
Side effects: –
• Hypersensitivity, gastrointestinal damage, hepatic toxicity and pancreatitis.
Pipobroman
Use:
• Against hematological cancers, especially polycythemia vera and chronic granulocytic leukemia.
Hormones
• Steroids hormones including estrogen, androgens, progestin and glucocorticoids act on the appropriate target tissues at the level of transcription.
• Target cells contain, in their cytoplasm specific protein receptors with very high affinities for the hormones.
• Binding of the hormone to the receptor transform the receptor structure, followed by migration of the resulting complex into the nucleus.
• In the nucleus, the complex interacts with an acceptor site to influence transcription.
• Normal and well – differentiated neoplastic target cells have a no. of hormone receptors and they depend on the hormones for stimulation.
• Less-differentiated neoplastic cells become independent of hormonal control and lose their specific receptors.
• Thus, some neoplasms are hormone dependent and responsive to hormone-based therapy, whereas others are independent and unresponsive
Miscellaneous Compounds
• Cisplatin, cisplatinum, or cis-diamminedichloroplatinum (II), is a well-known chemotherapeutic drug. It has been used for treatment of numerous human cancers including bladder, head and neck, lung, ovarian, and testicular cancers
Mitotane
• Mitotane is used to treat cancer of the adrenal gland that cannot be treated with surgery
• It works by slowing growth or reducing the size of the tumor
Immunotherapy
• Cells of neoplastic potential are continually produced in the human body and our immune surveillance system destroys them.
• The development of tumors implies that this system is not functioning properly.
• Evidence for this factors in carcinogenesis includes
✓ A high rate of cancer in organ – transplant patients whose immune systems are suppressed by drugs such as azathioprine.
✓ A high correlation between cancer and immune-deficiency diseases such as bacterial and viral infections.
✓ Stimulation of the body’s immune system is a valuable method of cancer treatment because it can eradicate the neoplastic cells completely.
• Interferons are secreted by cells in response to viral infections or other chemical or biological inducers.
• Three major classes of interferons – α, β, γ were identified.
• They bind to specific high-affinity receptors on cell surfaces, which induces a sequence of intracellular events, including the indication of enzymes.
• This process causes enhancement of natural killer cell activity, inhibition of certain oncogenes and increased specific cytotoxicity of lymphocytes for target cells.
• Interferons alfa – 2a, alfa – 2b and alfa – n3 promote the immunological response to neoplastic cells which results in significant cytotoxicity.
• These are the drugs of choice for treatment of hairy cell leukemia.
• Also effective against cell cancer, multiple myeloma, melanoma, kaposi’s sarcoma (clinical trials)
Interferon Alfa – 2a:
• Highly purified protein containing 165 amino acids.
Used: – in the treatment of hairly cell leukemia and chronic myelogenous leukemia
Side effects: hypersensitivity, (contraindicated), Flu-like syndrome, consisting of fever, fatigue, myalgia, headache and chills, gastrointestinal and CNS symptoms.
Interferon Alfa – 2b:
• It is a highly purified protein
Use: – Used for the treatment of Hairy cell leukemia.
• In treating melanoma and renal cell carcinoma.
Side effects: – flu-like syndrome, CNS effects and cardiovascular effects including hypotension, arrhythmia or tachycardia