Delamination of Glasses

Glass Delamination
Glass delamination may be defined as the appearance of thin flexible flakes of glass (lamellae or platy) with typical size ranges between less than 50 µm to 200 µm in a drug product solution.
Glass delamination is potentially a serious quality issue and may result in the recall of a product by the FDA.
The presence of glass lamellae is an indication of a strong interaction between the drug product in solution and the inner surface of the glass container.
Delamination of Glass
• Delamination of pharmaceutical glass is a serious issue, as it can cause glass particles to appear in product. In Type I pharmaceutical glass vials, delamination occurs generally at the bottom and shoulder, where extensive flaming during the conversion process can favor a strong evaporation of boron and sodium and the formation of heavily enriched silica layers.
• Effect of delamination:
q Contamination of products and market recall.
q Damage reputation of the drug manufacturer.
q Huge financial lose
Causes of Delamination
q Formulations with a high pH include phosphate and citrate buffers increase the risk of glass delamination.
q High alkali content in glass could accelerate erosion.
q High temperature during the vial-forming process increase the risk of glass delamination.
q Terminal sterilization (irradiated at 20-40 kGy [kilogray] for 150 min) also is a risk factor for specific products (veterinary parenteral administration), could cause delamination.
q High product-storage temperatures and long exposure times can increase the rate and severity of glass delamination.
Delamination Study under Electron Microscope
Scanning Electron Microscopy (SEM) is a very powerful tool for delamination studies and other glass defect investigations.
This is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons.
The electrons interact with atoms in the sample, producing various signals that contain information about the sample’s surface topography and composition.
Ref USP: USP<1660> Chapter (“Evaluation of the Inner Surface Durability of Glass Containers”) which recommends approaches to predict potential formation of glass particles and delamination.
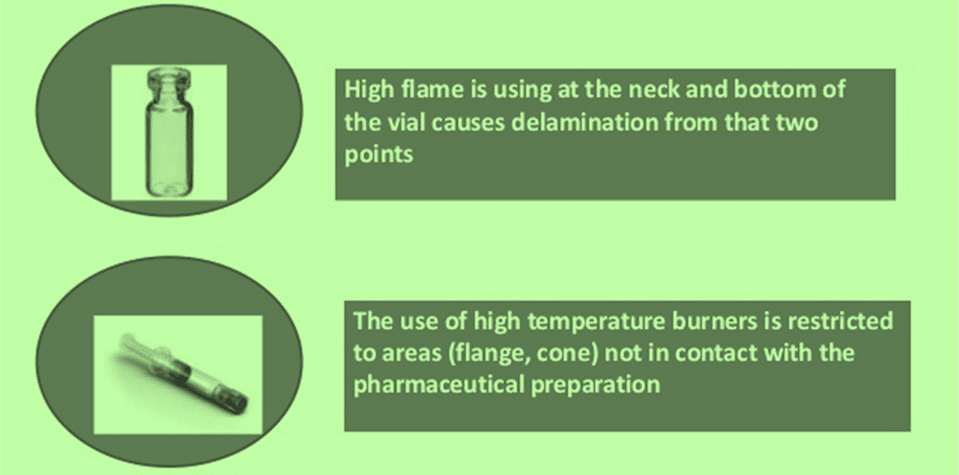
Difference in Delamination (Glass Vial Vs Syringe)
HOW CAN WE PREVENT DELAMINATION?
|
Option 1 |
Treating the surface of the glass vials with materials, such as |
|
Option 2 |
Polyglycerol coatings of glass vials for protein resistance |
|
Option 3 |
Consider alternative sterilization methods only in rare cases. |
|
Option 4 |
The correct specification for the glass to ensure its suitability for |
|
Option 5 |
The processing involved sterilization and autoclaving processes. Both |
|
Option 6 |
Glass manufacturers ensuring that appropriate process controls are in |
|
Option 7 |
Forming temperatures and correct annealing and due diligence in the |
|
Option 8 |
Durability analyses are conducted to assess the compatibility of the |
|
Option 9 |
Use COC/COP vial/syringe/cartridge. |
|
Option 10 |
Siliconization on inside the glass surface. |
Also, Visit: Packaging Materials in Pharmaceutical Industry