Distillation

Learning objectives
At the end of this Notes student will be able to:
- Define distillation and various terms associated with distillation
- Explain the theories of distillation with equations
- State the pharmaceutical applications of distillation
- Compare distillation with other unit operations
- Draw the diagram of a typical distillation unit
- Enlist the various types of distillation methods
- Describe operation of simple and steam distillation
- List the applications of simple and steam distillation
Distillation
It is an unit operation which involves separation of a vaporizable component from a multi-component system and subsequent condensation of vapours.
It is a process of separating the component substances from a liquid mixture by selective evaporation and condensation.
It is defined as the separation of the components of a liquid mixture by a process involving vaporization and subsequent condensation at another place.
It results in essentially complete separation (nearly pure components).
It can be applied for two immiscible or non-reacting solid and liquid or liquid and liquid.
Introduction to Distillation System
For any liquid, the individual molecules within the liquid are continuously in motion
A small percentage of these molecules attain enough kinetic energy to leave the liquid phase This exerts an opposing pressure on the atmosphere above the solution known as the vapor pressure, P

When enough energy, in the form of heat, is imparted to the solution the vapor pressure becomes equal to the atmospheric pressure and the liquid begins to boil

The vapor obtained from a boiling liquid, once cooled, will re-condense to a liquid known as the distillate The complete process is called a distillation

Applications
- Separation of volatile oils
- Purification of organic solvents
- Manufacture of official preparations
- Refining of petroleum products
- Recovery of solvents
- Quality control methods
- Separation of drugs obtained from plant and animal sources
- Purification of drugs obtained from chemical process
Differences between Distillation, Drying and Evaporation
| Distillation | Drying | Evaporation |
| Vaporization of bulk liquid above boiling point | Process of removal of the liquid solvent | Evaporation Vaporization of bulk liquid below boiling point |
| Product desired is distillate(solvent used) | Product desires is Solid residue (dried solute) | Product desires is concentrated liquid (concentrated solute) |
| Process of separation of the components of liquid mixture | Slow evaporation of wet solid bulk (small amount of liquid) | Process of complete removal of the liquid solvent |
Theory of Distillation
Distillation is a process of separating and purifying the components in a liquid mixture.
The primary data required to solve any distillation problem are vapour-liquid equilibrium relationship. Distillation method depends on the relative volatilities of the components present in the mixture.
Henry’s Law
Henry’s law is one of the gas laws and was formulated by the British chemist, William Henry, in 1803. It states that: “At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid”.
The vapour pressure of a liquid at a particular temperature is the equilibrium pressure exerted by molecules leaving the liquid surface.
Here are some important points regarding vapour pressure:
- Energy input raises vapour pressure
- Vapour pressure is related to boiling
- A liquid is said to ‘boil’ when its vapour pressure equals the surrounding pressure
- The ease with which a liquid boils depends on its volatility
- Liquids with high vapour pressures (volatile liquids) will boil at lower temperatures
- The vapour pressure and the boiling point of a liquid mixture depends on the relative amounts of the components in the mixture
Boiling point composition curves of mixture
- Since in distillation, repeated vaporisation and condensation processes are involved, simultaneously, the composition of liquid and vapour phases change continuously.
- Hence boiling point-composition curves are helpful in predicting whether the separation is possible or not.
- If possible, whether it is easy or difficult.
- These are helpful in designing the equipment for distillation.
The boiling point diagram shows how the equilibrium compositions of the components in a liquid mixture vary with temperature at a fixed pressure. Consider an example of a liquid mixture containing 2 components (A and B) – a binary mixture.
The following boiling point diagram:
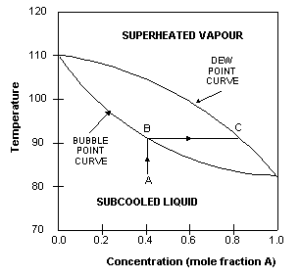
*The dew point is the temperature to which, the airborne water vapor will condense to form liquid water (dew). *bubble point is the temperature (at a given pressure) where the first bubble of vapor is formed
The boiling point of A is that at which the mole fraction of A is 1. The boiling point of B is that at which the mole fraction of A is 0. In this example, A is the more volatile component and therefore has a lower boiling point than B. The upper curve in the diagram is called the dew-point curve while the lower one is called the bubble-point curve.
The dew-point is the temperature at which the saturated vapour starts to condense. The bubble-point is the temperature at which the liquid starts to boil.
- The region above the dew-point curve shows the equilibrium composition of the superheated vapour while the region below the bubble-point curve shows the equilibrium composition of the sub cooled liquid.
- For example, when a sub cooled liquid with mole fraction of A=0.4 (point A) is heated, its concentration remains constant until it reaches the bubble-point (point B), when it starts to boil. The vapours evolved during the boiling has the equilibrium composition given by point C, approximately 0.8 mole fraction A. This is approximately 50% richer in A than the original liquid.
- This difference between liquid and vapour compositions is the basis for distillation operations.
Relative volatility
- Relative volatility is a measure of the differences in volatility between 2 components, and hence their boiling points. It indicates how easy or difficult a particular separation will be. The relative volatility of component ‘i’ with respect to component ‘j’ is defined as
α ij = [ yi/xi ] / [ yj/xj ]
yi = mole fraction of component ‘i’ in the vapour
xi = mole fraction of component ‘i’ in the liquid
Thus if the relative volatility between 2 components is very close to one, it is an indication that they have very similar vapour pressure characteristics.
This means that they have very similar boiling points and therefore, it will be difficult to separate the two components via distillation.
Vapour Liquid Equilibria
Distillation columns are designed based on the boiling point properties of the components in the mixtures being separated.Thus the sizes, particularly the height, of distillation columns are determined by the vapour liquid equilibrium (VLE) data for the mixtures.
Vapour-Liquid-Equilibrium (VLE) Curves

Constant pressure VLE data is obtained from boiling point diagrams. VLE data of binary mixtures is often presented as a plot, as shown in the figure on the right. The VLE plot expresses the bubble-point and the dew-point of a binary mixture at constant pressure.
The curved line is called the equilibrium line and describes the compositions of the liquid and vapour in equilibrium at some fixed pressure.
This particular VLE plot shows a binary mixture that has a uniform vapour-liquid equilibrium that is relatively easy to separate.
Vapor-Liquid Equilibrium Relations
Binary mixture
When two liquids are mixed together, they may be miscible with each other in all proportions. Such miscible mixtures are known as binary mixture (ethyl alcohol and water, water and acetone, benzene and carbon tetrachloride.
Ideal solutions
One in which there is no change in the properties of the components other than dilution, when they are mixed to form a solution.
Raoult’s Law
“The partial vapour pressure of each volatile constituent is equal to the vapour pressure of the pure constituent multiplied by its mole fraction in the solution at a given temperature”.
It expresses a quantitative relationship between the concentration and vapour pressure.
Consider a mixture of miscible liquids A and B. In this mixture
Let the partial vapour pressure exerted by A = PA kPa
Let the partial vapour pressure exerted by B = PB kPa
Let the vapour pressure exerted by the pure component A = PO A kPa
Let the vapour pressure exerted by the pure component B = PO B kPa
Let mole fraction concentration of liquid A = XA
Let mole fraction concentration of liquid B = XB
Raoul’s Law Mathematically expressed as
Partial vapour pressure of a liquid = Vapour pressure of a pure liquid X mole fraction of a liquid
PA = PO A XA
PB = PO B XB
- A mixture of ethylene chloride and benzene obeys Raoult’s law when two liquids are mixed, the vapour pressure of each one is reduced by the pressure of other to the extent of dilution of each phase.
- Ideal solution is defined as the one that obey’s Raoults law
- This law is obeyed by only a few solutions of liquid in liquids (perfect solutions)
Benzene and toulene, n hexane and n heptane, ethyl bromide and ethyl iodide
Real solutions and deviations
Most systems show varying degree of deviations from Raoult’s law, depending on the nature of the liquids and the temperature. These solutions are known as Real solutions.
Deviations are observed because solute-solute, solvent-solute and solvent-solvent interactions are unequal. Examples include carbon tetrachloride and cyclohexane, chloroform and acetone.
Positive deviation
The vapour pressure is greater than that of the sum of the partial pressures of the individual components. (Carbon tetrachloride and cyclohexane, benzene and ethanol)
Negative deviation
The vapour pressure is lower than that of the sum of the partial pressures of the individual components (chloroform and acetone, water and nitric acid)
Different Parts of Distillation

Typical Distillation Setup

Various Types of Distillation
- Simple Distillation
- Molecular Distillation
- Vacuum Distillation
- Steam Distillation
- Compression Distillation
- Flash Distillation
- Fractional Distillation
- Azeotropic and extractive Distillation
- Destructive distillation
SIMPLE DISTILLATION
- Simple distillation is a process of converting a single constituent from a liquid (or mixture) into its vapour, transferring the vapour to another place and recovering the liquid by condensing the vapour, usually by allowing it to come in contact with a cold surface.
- This process is known differential distillation, as distillation is based on the differences in volatilities and vapour pressures of the components in the mixture.
- Principle: Liquid boils when its vapour pressure is equal to atmospheric pressure. Simple distillation is conducted at its boiling point. The higher the relative volatility of a liquid, the better is the separation by simple distillation. Heat is supplied to the liquid so that it boils. The resulting vapour is transferred to a different place and condensed.
Assembling of apparatus
The construction of a simple distillation apparatus is shown in Figure. It consists of a distillation flask with a side arm sloping downwards.
Condenser is fitted into the side arm by means of a cork.
The condenser is usually water condenser, i.e., jacketed for circulation of water. The condenser is connected to a receiver flask using an adapter with ground glass joints.
On a laboratory scale, the whole apparatus is made of glass.
Simple Distillation Process
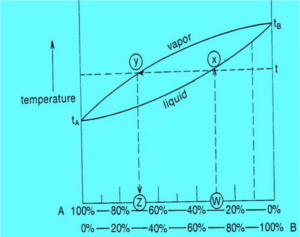
- When a mixture AB of a specific composition is heated, the total vapor pressure (composed of the contributions of PA and PB) will rise until it is equal to the external vapor pressure.
- The mixture will begin to boil.
- Assume a two component mixture with a composition of 30%A:70%B (point W). The boiling point of this mixture is found by drawing a vertical line from W to where it intersects the lower curve (point X).
- A horizontal line drawn from X to where it intersects the vertical axis (the temperature) gives the boiling point of composition W.
- From the point (X) where this horizontal line intersects the upper curve (vapor) drop a vertical line to intersect the lower axis (the composition).
- Point Z gives the composition of the vapor which is in equilibrium with a liquid of composition W at its boiling point.
Procedure
The liquid to be distilled is filled into the flask to one-half to two-third of its volume.
Bumping is avoided by adding small pieces of porcelain before distillation.
A thermometer is inserted into the cork and fixed to the flask.
The thermometer bulb must be just below the level of the side arm.
Water is circulated through the jacket of the condenser.
The contents are heated gradually. The liquid begins to boil after some time.
The vapour begins to rise up and passes down the side arm into the condenser.
The temperature rises rapidly and reaches a constant value.
The temperature of the distillate is noted down, which is equal to the boiling point of the liquid.
The vapour is condensed and collected into the receiver.
The flame is adjusted so that the distillate is collected at the rate of one to two drops per second. Distillation should be continued until a small volume of liquid remains in the flask.
Applications:
(I) Simple distillation is used for the preparation of distilled water and water for injection
(2) Volatile and aromatic waters are prepared
(3) Organic solvents are purified
(4) A few official compounds are prepared by distillation Examples are spirit of nitrous ether and aromatic spirit of ammonia
(5) Non-volatile solids are separated from volatile liquids
Steam Distillation
Steam distillation is used for separating substances which are immiscible with water, volatile in steam & having high vapour pressure at the boiling temperature of water
It is a method of distillation carried with the aid of steam and used for the separation of high boiling substance from non-volatile impurities
It is the most common example of differential distillation
Principle
- A mixture of immiscible liquids begins to boil when the sum of their vapor pressure is equal to the atmospheric pressure.
- In case of a mixture of turpentine oil and water, mixture boils below the BP of water, though the turpentine boils at a much higher temperature than that of water.
- For example, the BP of turpentine is about 1600C. on mixing with water and heated the mixture boils at about 95.60C
- At this temperature, the VP of water is 86.24 kPa (647 mm Hg) and that of turpentine is 15.05 kPa (113 mm Hg)
- The sum of VPs is 101.31 kPa (760 mm Hg) which is normal AP.
- Thus high boiling substances can be distilled at a temperature much below its BP when steam is used
Construction

- It consists of a metallic ‘steam can’ fitted with a cork having two holes.
- Through one of the holes, a long tube is passed so as to reach almost the bottom of steam generator.
- This tube acts as a safety tube, so that in case the pressure inside the steam generator becomes too much, water will be forced out of it and the pressure will be relieved. Moreover, when steam start coming out from the safety tube; it indicates that the steam can is almost empty.
- Through another hole a bent tube is passed.
- Another end of the bent tube is connected to the flask containing non-aqueous liquid (for example, crude containing volatile oil) through a rubber bung. The tube should reach almost the bottom of the flask.
- Through the other hole of the rubber bung, a delivery tube is inserted which connects the flask and the condenser.
- The condenser is connected to a receiver flask using an adaptor.
Provisions are made to heat the steam can and flask.
Working of steam Distillation
The non- aqueous liquid is placed in the flask. A small quantity of water is added to it. Steam can is filled with water.
The steam generator and the flask are heated simultaneously, so that a uniform flow of steam passes through the boiling mixture. The mixture gets heated.
The steam carries the volatile oil and passes into the condenser, which is cooled by cold water.
The condensed immiscible liquid is collected into receiver. Distillation is continued until all the non- aqueous liquid has been distilled. In the receiver, water and organic liquid form two separate layers, which can be easily separated using a separating flask.

Florentine Receiver

Applications
- It is used for the separation of immiscible liquids (toulene and water)
- Extracting most of the volatile oils (anise, clove and eucalyptus)
- Purification of liquid with high BP (essential oil of almond)
- Camphor can be distilled
- Aromatic water are prepared by this method
Flash Distillation
Flash distillation is defined as a process in which the entire liquid mixture is suddenly vaporized (flash) by passing the feed from a high pressure zone to a low pressure zone. Flash distillation is also known as equilibrium distillation, i.e., separation is attempted when the liquid and vapour phases are in equilibrium. This method is frequently carried out as a continuous process.
Principle
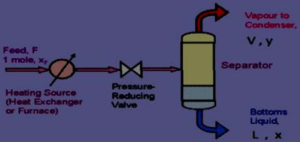
- When a hot liquid mixture is allowed to enter from a high-pressure zone into a low-pressure zone, the entire liquid mixture is suddenly vaporised. This process is known as flash vaporisation. During this process the chamber gets cooled.
- The individual vapour phase molecules of high boiling fraction get condensed, while low boiling fraction remains as vapour.
Working
- The feed is pumped through a heater at a certain pressure. The liquid gets heated, which enters the vapour-liquid separator through a pressure-reducing valve. Due to the drop in pressure, the hot liquid flashes, which further enhances the vaporisation process.
- The sudden vaporisation induces cooling. The individual vapour phase molecules of high boiling fraction get condensed, while low boiling fraction remains as vapour. The mixture is allowed for a sufficient time, so that vapour and liquid portions separate and achieve equilibrium.
- The vapour is separated through a pipe from above and liquid is collected from the bottom of the separator. By continuously feeding into the still, it is possible to obtain continuous flash distillation.
- The operating conditions can be adjusted in such a way that the amount of feed exactly equals the amount of material removed. Therefore, vapour and liquid concentrations at any point remain constant in the unit.

Uses: Flash distillation is used for separating components, which boil at widely different temperatures. It is widely used in petroleum industry for refining crude oil.
Advantages: Flash distillation is a continuous process.
Disadvantages: Flash distillation is not effective in separating components of comparable volatility. It is not an efficient distillation when nearly pure components are required, because the condensed vapour and residual liquid are far from pure.
Azeotropic and Extractive Distillation
- An azeotrope is a mixture of two or more liquids (chemicals) in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture.
- Because their composition is unchanged by distillation, azeotropes are also called constant boiling mixtures.
- Positive azeotrope is 95.63 % ethanol and 4.37 % water (by weight). Ethanol boils at 78.4 °C, water boils at 100 °C, but the azeotrope boils at 78.2 °C, which is lower than either of its constituents.
- Negative azeotrope is hydrochloric acid at a concentration of 20.2 % and 79.8 % water (by weight). Hydrogen chloride boils at -84°C and water at 100°C, but the azeotrope boils at 110°C, which is higher than either of its constituents.
Now if in distillation azeotrop forms then we can’t separate them so we add foreign material which is known as ENTRAINER. We choose the entrainer in such a manner so that it can make a minimum boiling azeotrope with one of the present component.
- For Ex:- A+B make a azeotropes and we use C as a entrainer.
- C makes a minimum boiling azeotropes with A
- Now if we start distillation then C+A behave as a one component and B as other
- So we find C+A in distillate because it makes a minimum boiling azeotropes and B as a bottom product • So in this way you can separate azeotropes by distillation
- For example:
- Benzene is added to the azeotropic mixture of water and ethyl alcohol. Benzene breaks the mixture water-ethyl alcohol and form new azeotrope between benzene and ethyl alcohol.
- The volatility of water is enhanced. On distillation, water distills at 65.85 °C leaving EA and benzene behind.
- Boiling point of this binary mixture is 68.2 °C and benzene gets distilled leaving pure alcohol behind. It can be distilled off at 78.3 °C.
- In Extractive distillation, the third substance added to the azeotropic mixture is miscible, has high boiling point and relatively non-volatile liquid compared to the components to be separated.
- The solvent interacts with the components of the mixture thereby causing their relative volatilities to change.
- Example: Separation of toluene from paraffin hydrocarbons of approximately same molecular weights. The separation of toluene and isooctane (example of hydrocarbon) is difficult.
- In the presence of phenol, the relative volatility of isooctane increases, therefore, separation of toluene is relatively easy.
Applications
The liquor from fermentation process is a common source of ethanol and contains approximately 8–10%. Petroleum refineries and distilleries use these types of distillation
Differences
- Azeotropic distillation is a process of distillation wherein you can add a certain component into the mixture to have a better separation process. Usually, the certain component added into the mixture is water or benzene, because these can help in increasing the volatility of a substance.
- Extractive distillation is a distillation technique wherein the capability of mixing or miscibility, the component of being non-volatile, and even a high boiling point, could be the measurement of separating a mixture without even forming an azeotrope. This kind of method is usually used for mixtures with almost the same volatility.
Molecular Distillation: Definition
- It is a distillation process in which each molecule in the vapour phase travels mean free path and gets condensed individually without intermolecular collisions on application of vacuum
- Molecular distillation is based on the principle of the simple distillation with some modifications
- This is also called evaporation distillation or short path distillation
Principle
- The substances to be distilled have very low vapour pressures. Examples are viscous liquids, oils, greases, waxy materials and high molecular weight substances. These boil at very high temperature.
- In order to decrease the boiling point of the liquids, high vacuum must be applied
- The vapour pressure above the liquid is much lower.
- At very low pressure, the distance between the evaporating surface and the condenser is approximately equal to the mean free path of the vapour molecules
- Molecules leaving the surface of the liquid are more likely hit the condenser surface nearby. Each molecule is condensed individually
- The distillate is subsequently collected
Theory
The mean free path of a molecule is defined as the average distance through which a molecule can move without coming into collision with another
The mean path (λ.) can be expressed mathematically as:
λ = η v 3 / p.ρ
where. p = vapour pressure, kPa
p = density, kg/m3
η = viscosity, Pa’s
λ = mean path length, m
For example, mean path (heavy molecules) of butyl phthalate is about 30 mm and of olive oil is 20 mm when measured at a pressure of 0.1 pascal
The mean free path can be increased by decreasing the viscosity which can be obtained at high temperature and low pressure
Thus, nonvolatile substances may become volatile and distillation is possible
Requirements for design the equipment
- The evaporating surface must be close to the condensing surface.
This ensures the molecules to come in contact with the condenser as soon as they leave the evaporating surface
For this reason, this process is also known as short path distillation
- The molecular collisions should be minimized because they change the direction of the path of molecules
- In other words, intermolecular distances should be fairly high.
- It can be achieved under very high vacuum, usually of the order of 0.1 to 1.0 pascals
- The liquid surface area must be as large as possible as so that the vapour is evolved from the surface only, but not by boiling.
- Thus this process is also called evaporation distillation
Centrifugal Molecular Still
Principle
- In this method, liquid feed is introduced into a vessel, which is rotated at very high speed (centrifugal action)
- On account of heating, vaporisation occurs from a film of liquid on the sides of the vessel
- The vapour (molecules) travels a short distance and gets condensed on the adjacent condenser
- Each molecule is condensed individually
- The distillate is subsequently collected
Construction

- It consists of a bucket-shaped vessel having a diameter of about 1 to 1.5 m
- It is rotated at high speed using a motor
- Radiant heaters are provided externally to heat the fluid in the bucket
- Condensers are arranged very close to the evaporating surface.
- Vacuum pump is connected to the entire vessel at the top
- Provisions are made for introducing the feed into the centre of the bucket, for receiving the product and residue for re-circulation
Working
- Vacuum is applied at the centre of the vessel. The bucket shaped vessel is allowed to rotate at high speed • The feed is introduced from the centre of the vessel
- Due to centrifugal action of the rotating bucket, liquid moves outward over the surface of the vessel and forms a film • Since, the radiant heaters heat the surface, the liquid evaporates directly from the film
- The vapour (molecules) travels its mean tree path and strikes the condenser. The condensate is collected into another vessel
- The residue is collected from the bottom of the vessel and is recirculated through the feed port for further distillation
Applications
Molecular distillation is used for the purification and separation of chemicals of low vapour pressure 1. Purification of chemicals such as tricresyl phosphate, dibutyl phthalate and dimethyl phthalate 2. More frequently used in the refining of fixed oils
- Vitamin A is separated from fish liver oil. Vitamins are concentrated by this method from fish liver oils and other vegetable oils
- Free fatty acids are distilled at 100°C
- Steroids can be obtained between 100°C and 200°C, while triglycerides can be obtained from 200°C onwards.
Summary
- Distillation is an unit operation used for the complete separation of a liquid mixture by vaporization and subsequent condensation
- Vapour-liquid equilibrium relationship data is required to solve any distillation problem
- Distillation depends on the relative volatilities of the components present in the mixture
- Henry’s law states that the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid
- Raoult’s law expresses a quantitative relationship between the concentration and vapour pressure • Ideal solutions are the one which obeys and real solutions shows varying degree of deviations from Raoult’s law
- Distillation is used for the separation of volatile oils, purification of organic solvents, recovery of solvents and purification of drugs obtained from chemical process and natural sources
- Basic parts of a typical distillation set up are still, condenser and receiver with a boiler
- Various distillation methods include simple, steam, flash, azeotropic and molecular
- Simple distillation or differential is based on the differences in volatilities and vapour pressures of the components in the mixture
- Simple distillation is conducted at its boiling point where liquid boils when its vapour pressure equals atmospheric pressure and used for the preparation of distilled water and water for injection
- Steam distillation, simplest form of differential distillation is used for separating substances which are immiscible with water with the aid of steam and used for the separation of high boiling substance from non-volatile impurities. Camphor can be distilled by this method
- Flash distillation utilizes difference in pressure zone for sudden vaporization of entire liquid mixture and carried out as a continuous process and does not involve rectification
- An azeotrope is a mixture of two or more liquids whose composition cannot be changed by simple distillation. As their composition is unchanged by distillation, azeotropes are also called constant boiling mixtures
- Extractive distillation is carried out in the presence of a miscible, high boiling, relatively non-volatile component, and the solvent.
- Molecular distillation or evaporation distillation or short path distillation is based on the principle of the simple distillation with some modifications and utilizes the concept of mean free path
For Detailed PDF Notes Click on Download Button
