Antimalarial agents
Contents
• Malaria – Etiology
• Life cycle of Malaria parasite
• Modern malaria chemotherapy
• Classification of Antimalarials
• Structural Activity Relationship, pharmacokinetics,
pharmacological effects and side effects of the following:
Cinchona
alkaloids
8-
aminoquinolines
4-
aminoquinolines
9- acridines
• Synthesis of chloroquine
• Synthesis of primaquine
• Synthesis of quinacrine
• Biguanides – biological transformation, SAR, metabolism,
effects and side effects
• Pyrimidines – site of action, SAR
• Sulphones and sulphonamides – Action and toxicity
• Mechanism of action of Antimalarials
Learning
Objectives
At the end of this lecture, student will be able to
• Discuss the etiology of Malaria
• Explain modern malarial therapy
• Classify Antimalarials
• Compare the structure and activity relations of
Antimalarials
• Discuss the metabolism, effects and side effects of
Antimalarials
• Explain the synthetic route for the synthesis of
chloriquine
• Explain the synthetic route for the synthesis of
primaquine
• Explain the synthetic route for the synthesis of
quinacrine
• Compare the structure with that of activity of
antimalarials
• Discuss the biotransformation of specified antimalarials
• Describe the action and toxic effects of antimalarials
• Explain the synthesis of certain antimalarials
Etiology
Etiology (study of the cause/ causation of disease or
condition):
Malaria in humans is caused by four species of Plasmodium
(protozoan parasite)
• Plasmodium Vivax (benign tertian malaria)
• Plasmodium falciparum (malignant tertian, sub-tertian
malaria)
• Plasmodium malariae (quartan malaria)
• Plasmodium ovale (mild tertian malaria ovale tertian)
In other mammals, birds and reptiles it is caused by many
other species.
Symptoms
• The disease is characterized by successive chills, fever
and sweats.
• If the symptoms occur every 3rd day- Tertian, if it occurs
every fourth day- Quartan
• All species of plasmodium have two hosts, a vertebrate and
a mosquito that acts as both vector (carrier) and a definitive host.
• Vector for human malaria- Female ‘Anopheles’ Mosquitoe
• The sexual phase of life cycle begins when a female
mosquito bites an infected
vertebrate and ingests blood containing the malarial parasite in the gametocyte
stage.
Life cycle
of Malarial parasite
• Mosquito bites an infected vertebrate and ingests the
malarial parasite
• In the stomach
of the mosquito,
the sexual phase
of development called sporogony
occurs.
• The male-female gametocytes form gametes. An ookenite
(zygotes) is formed by fertilization and penetrates the stomach wall outside
the stomach wall. Outside the stomach, oocysts are formed which produces
sporozoites, that are released by the rupturing of the oocyst.
• The sporozoites travel to the salivary glands of the
mosquito, from which they may be transferred to an uninfected vertebrate host
by the bite of mosquito.
• Injected sporozoites disappear rapidly from the blood of
the vertebrate, entering the parenchyma cells of the liver and some other
tissues. The parasite now begins
the asexual phase
of development called schizogony.
• In this pre-erythrocytic stage, the parasite grows and
divides to form schizont. The schizont segment to form many merozoites, which
causes the rupturing of the cell, beginning the erythrocytic stage.
• Within the red blood cells, the merozoites become
trophozoites and multiplication occurs by schizogony .
• The schizonts formed from the trophozoites divide into
merozoites and continuously increase the no.of merozoites available to invade
more red blood cells, so that, finally the no. of rupturing cells is
sufficiently great to initiate the symptoms of disease.
• The asexual cycle continues until chemotherapy is
initiated, immunity is developed or death occurs.
• ‘The continuous invasion and subsequent rupture of
erythrocytes lead to the development of another significant symptoms of
malaria, anemia.
• When normal reproduction of the erythrocytes becomes
unfavorable, some trophozoites from the erythrocyte stage develop into male or
female gametocytes, which circulate in the blood to be available for ingestion
by another mosquito.
Potential
ways to control malaria
• Elimination of the
vector-simplest, cost effective
Prevent contact with insect- a nocturnal feeder-use window
screens, mosquito nets
Eliminate mosquito by application of insectidies &
destroy breeding grounds
• Drug
therapy-tremendous need for new, more effective drugs
Cause protozoa develop resistance by different mechanisms
& there are a variety of adverse reactions. No single drug is effective
against all species
• Vaccination- no
effective vaccination has been developed
The parasite does not elicit an effective immune
response. The only approved vaccine as
of 2015 is RTS,S,-trade name Mosquirix. It has relatively low efficacy.
Possible
sites for drug therapy
• Kill the sporozoites injected by the mosquito and/or
prevent the sporozoites from entering the body
• Kill the primary schizonts in the hepatocytes and/or
prevent them form becoming merozoites
• Kill the merozoits in the blood and/or prevet them from
developing into gametocytes
• Kill the gametocytes before they enter the mosquito and
fertilize into zygotes
Modern
malaria chemotherapy
• Most drugs used in modern malarial chemotherapy as
chloroquine, amodiaquine, pyrimethamine, quinine, sulfonamides act primarily at
the erythrocytic stage, in the malaria life cycle (i.e. at site 4). Since the
severe and life threatening clinical symptoms of malaria occur at the stage,
these drugs are very useful in
1. Treating all four
human malarias and
2. In preventing
clinical symptoms of four human malarias.
• However, cures from these ‘site-4’ drugs can result only
with P. falciparum – the other three species P. vivax, P. malariae and P. ovale
have a ‘secondary exo-erythrocyte’ (secondary schizont) stage which can
periodically release new merozoites for years or decades.
• An additional drug which is effective at the ‘site-3’
stage is usually primaquine.
• It is desirable to protect humans from initial infection
by the mosquito at ‘site one’. But no
drugs are available which are effective at this site.
• Primaquine is active at ‘site 2’, so it could be used as a
prophylactic against all forms of human malaria, but due to its toxicity it
cannot be used for a prolonged period.
• Primaquine is also effective as a gametocide (at site 5)
• The best means of controlling the spread of the disease is
through community sanitation and use of insecticide.
• A complicating factor in modern malaria chemotherapy is
that drug- resistant strains of plasmodia have been reported. Eg. Chloroquine
resistant P. falciparum or P. vivax in one geographical area and quinine
resistant P. falciparum in another.
Classification
of Antimalarials
The important classes of antimalarial drugs are:
Cinchona alkaloids
4-aminoquinolines
8-aminoquinolines
9-aminoacridines
Biguanides
Pyrimidines (diaminopyrimidines)
Sulfones
Newer antimalarials
• Cinchona alkaloids
Quinine
Quinidine
Cinchonine
Cinchonidine
• 4-aminoquinolines
Chloroquine
Hydroxychloroquine
Mefloquine
Amodiaquine
• 8-aminoquinolines
Primaquine
Pamaquine
Pentaquine
• 9-aminoacridines
Quinacrine
Acriquine
• Biguanides
Proguanil
Chloroproquanil
Cycloguanil
• Pyrimidines
Pyrimethamaine
Trimethoprim
• Sulphones
Dapsone
• Polycyclic
antimalarial drugs
Doxycycline
Halofantrine
• Newer antimalarials
Artesunate
Artemether
Atovoquon
Cinchona
alkaloids
Eg. Quinine, Quinidine, Cinchonine, Cinchonidine.
The alkaloids are derivatives of 4-quinolinemethanol bearing
a substituted quinuclidine ring system
SAR of Cinchona
alkaloids
1. All four of the cinchona alkaloids are active
antimalarials. Thus the 6-OCH3 group is not essential for activity.
2. The quinoline methanol portion becomes important in
synthetic drugs.
3. All the alkaloids having same configuration at R1 &
R2 are diastereoisomers, differing in configuration at 3rd & 4th chiral
centers (C-8 and C-9)
4. Although all four alkaloids show antimalarial activity,
their C-9 epimers (i.e. having either 8R:9R or 8S:9S configurations) are
inactive.
5. Any modification of the 2˚ alcohol at C-9, through
oxidation, esterification and similar processes diminishes activity.
6. The quinuclidine portion is not essential for activity;
however, the tertiary (3˚) alkyl amine attached to C-9 is important. This forms
the basis for the design of synthetic antimalarials.
Metabolism: –
• Quinine is metabolized in the liver to the 2’ – hydroxyl
(carbostyril) derivative, followed by additional hydroxylation on the
quinuclidine ring to provide the 2,2’-dihydroxy Derivative as the major
metabolite.
• This metabolite has low antimalarial activity and is
rapidly excreted.
• Excretion is mainly in the urine.
Effects: –
• The cinchona alkaloids act on the erythrocytic merozoites.
They do not effect a radical cure but decrease symptoms.
• Quinine is used in treating some forms of malaria, in
which resistance to other agents has developed.
• Also, cinchona alkaloids are antipyretic by the action on
central temperature regulating mechanism causing peripheral vasodilation
Side effects: –
• Side effects include – skin allergies, deafness, vertigo
(giddiness – dizziness) and slight mental depression.
• Quinine passes the fetal barrier and affects the vision of
the new born.
Advantages: –
• Quinine is the
drug of choice
only for chloroquine
resistant P. falciparum. The
resistance to quinine has not developed as readily as it has to the synthetic
drugs.
Quinoline
analogues – 4-aminoquinolines
Chloroquine
Hydroxychloroquine
Amodiaquine
Mefloquine
SAR of
4-aminoquinolones
• The 3˚ amine is important for activity
• Side chain length (4-carbon) & 7-chloro-group are
optimal for activity.
• Substitution of –OH gp on one of the ethyl group on the 3˚
amine reduces toxicity and increase the plasma concentrations (more effective)
– a metabolite of chloroquine (hydroxyl chloroquine).
• Incorporation
of an aromatic
ring at the
3˚ amino gp,
produces a compound of reduced
activity and toxicity e.g amodiaquine
• Incorporation of a methyl group on C-3 on the quinoline
ring decreases activity e.g santoquine
• Substitution of a methyl group on C-8 causes a complete
loss of activity.
Absorption,
Distribution and excretion:
• Chloroquine is absorbed readily from the G.I,T, but
amodiaquine gives lower plasma levels than others in the group.
• Peak plasma concentrations are reached in 1 to 3 hrs, with
blood levels falling off rapidly after administration is stopped.
• About half the drug in the plasma is protein bound.
• These drugs concentrate in the liver, spleen, heart,
kidney & brain.
• These compounds are excreted rapidly with most of the
unmetabolized drug being accounted for in the urine.
Uses
• These drugs are active against the erythrocytic forms of
all malarial parasites leading to clinical cure.
• They do not prevent the disease and they are not active
against the liver infecting forms.
• They are also used in the treatment of extra-intestinal
amebiasis.
Toxicity
• The toxicity of 4-amino quinolone is quite low. The side
effects include nausea, vomiting, anorexia, abdominal cramps, diarrhea,
headache, dizziness, pruritus and urticaria
• Long-term administration in high doses may have serious
effects on the eyes.
• Patients with liver diseases particularly should be
watched when 4- aminoquinolines are used.
8-aminoquinolines
• 8-aminoquinolines, unlike 4-aminoquinolines, are active
against the pre- or exo-erythrocytic forms of the malarial parasite.
• 8-aminoquinolines are reserved for prophylactic purposes
and for the production of radical cure in infections due to P. vivax and P.
malariae
SAR: –
• The 6-methoxy group is essential for activity
• Side chain carbon length can vary from 4 to 6 carbons
• The extent of substitution of the amino is not as critical
and the drug of choice, Primaquine, is a primary amine.
Primaquine
Pamaquine
Pentaquine
ADME
• The 8-aminoquinolines are absorbed rapidly from the G. I
.tract. Peak plasma concentrations are reached within 2 hours after ingestion
after which the drug rapidly disappears from the blood.
• The drugs are localized mainly in the liver, lung, brain,
heart and muscle tissue.
• Metabolic changes are produced in the drug very rapidly
and on excretion, metabolic products account for nearly all of the drug.
• The antiplasmodial and the toxic properties of these drugs
are produced by metabolic transformation products.
Toxicity: –
• The toxic effects are principally in the CNS and the
hematopoietic system (system pertaining to the formation of blood cells).
• Other side effects are anorexia, abdominal pain, vomiting
and cyanosis (a dark bluish colaration of skin and mucous membrane due to
deficient oxygenation of the blood
in the tissues),
hemolytic anemia leukopenia
(abnormal decrease in WBC<5000/cu.mm) and methemoglobinemia (condition
in which more than 1% of hemoglobin is blood is oxidized to ferric form
Fe+++)
Uses: –
Primaquine is used mainly to prevent relapses due to exo-
erythrocytic forms of the parasites.
9-Aminoacridines
Act as schizonticides but are inferior to the
4-aminoquinolines
• Quinacrine hydrochloride (Mepacrine HCl)
• 6-chloro-9[{4-(diethylamino)-1-methylbutyl}amino]-2-methoxy
acridine dihydrochloride.
Toxicity: –
• Extremely toxic – largely replaced by the
4-aminoquinolines
• The toxicity involves the CNS, blood and fatal drug
reactions.
• The toxic effects
include-convulsions,
psychotomimetic (mental
disturbances) reactions, aplastic anemia [decreased formation of
erythrocytes and hemoglobin
from aplastic(defective) bone marrow] and exfoliate (scabial)
dermatitis
• A side effect of therapy is yellow pigmentation of the skin
and yellow color in the urine.
Synthesis
of chloroquine
• It is prepared by adopting the following four steps viz.,
• (a) Preparation of 4, 7-Dichloroquinoline (i.e., the
nucleus)
• (b)
Preparation of 2-amino-5-diethyl amino
pentane, or 1- diethylamino-4-amino pentane (i.e.,the
side chain).
• (c) Condensation of ‘a’ and ‘b’.
• (d) Addition of concentrated phosphoric acid to a hot
ethanolic solution of the condensed product.
A) Prep. of nucleus
B) Prep. of side
chain
C) Condensation of A
and B
D) Prep. of phosphate
salt
Synthesis of
Chloroquine
Synthesis
of Pamaquine
• Synthesis of the
nucleus
• Condensation of the
side chain and nucleus
• 8amino 6 methoxy quinolone reacts with 2 chloro diethyl
amino pentane to form pamaquine.
Synthesis
of Quinacrine
• Synthesis of the
nucleus
• Condensation with the side chain
Biguanides
• Biguanides are prodrugs for their active metabolites-the
dihydrotriazines (cyclized product)
• Biological transformation is illustrated with Proguanil
(chlorguanide).
• The antimalarial agent formed in this instance is the drug
cycloguanil, which itself is available as the pamoate salt, having a duration
of action of several weeks to months.
Biotransformation of
proguanil
SAR: –
• Substitution of a halogen on the para-position of the
phenyl ring significantly increases activity e.g. Chlorine substitution in
chloroguanil –
• The 4-bromine analog also is very active.
• A second chlorine at the 3-position of the phenyl ring of
proguanil further enhanced the activity.
• However, the dichloro compound, chlorproguanil, is more
toxic than chlorguanil.
Absorbtion,
distribution and elimination: –
• They are absorbed very quickly from the GIT.
• They are concentrated in the liver, lungs, spleen &
kidney but does not cross the blood brain barrier.
• 75% of the drug present in plasma is bound to protein
• They are metabolized and eliminated rapidly, mainly in the
urine
• As a result, frequent administration of these drugs is
necessary.
Toxicity: –
• Low toxicity, but with increased doses, haematuria (blood
in urine) and albuminuria (albumin in urine) are observed
Effects: –
• These derivatives including cycloguanil are potent
schizonticides against both exoerythroctic & erythrocytic forms of P.
falciparum and P. vivax.
• Resistance to these agents develops frequently.
Pyrimidines
Pyrimethamine
Trimethoprim
• The mechanism of action of pyrimidines is different and
their structures are not related to quinine and aminoquinolines.
• The sites of
action for these
derivatives include both
the erythrocytic forms of P. vivax.
MOA
• Structurally, these derivatives resemble the pteridine
portion of dihydrofolic acid (FH2)
• And interfere with its reduction to tetrahydrofolic acid (FH4)
by dihydrofolate reductase, thereby interfering with the utilization of folic
acid (in malarial protozoa)
• Pyrimethamine & trimethoprim are used in suppressive
treatment and as radical cure agents.
SAR: –
• Maximum activity is obtained when an electron-donating
group was present in the 6-position e.g alkyl.
• When a chlorine atom is present in the para-position of
the phenyl ring a maximum activity is obtained.
• If the two rings are separated by either an oxygen atom or
a carbon atom, antimalarial action is decreased. Eg. Trimethoprim
ADME: –
• Pyrimethamine is slowly but completely absorbed from the
G.I.T.
• It is localized in the liver, the lungs, the kidney &
the spleen.
• It is completely metabolized
• It is slowly excreted through the urine.
• Trimethoprim has a shorter half-life (24hrs) than
pyrimethamine.
Toxicity: –
• Pyrimethamine is relatively nontoxic, but overdoses may
lead to depression of cell growth by inhibition of folic acid activity.
Sulfones
• Dapsone:- 4,4’- diaminodiphenyl sulfone has a prophylactic
activity against resistant P. falciparum.
• It is was developed for the treatment of leprosy.
• Dapsone act by competing with PABA, in the synthesis of
folic acid.
• Dapsone has a prolonged duration of action and a moderate
toxicity. Combination with pyrimethamine have been effective in suppressing
symptoms of malaria due to chloroquine-resistant P. falciparum.
Sulfonamides
• Sulfonamides are used in antimalarial therapy against
drug- resistant malarial strains.
• They are effective against erythrocytic stages of the
malarial protozoa.
• Medium or long-acting sulfonamides are used clinically as antimalarials
particularly sulfadiazine, sulfadoxine and sulfalene.
• Each of the above sulfonamides are much more effective
when given in combination with pyrimethamine.
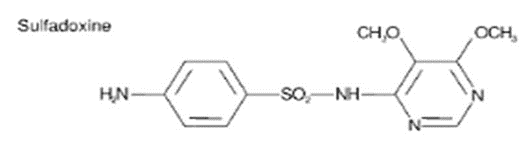
Sulfadoxine: –
Sulfalene: –
Sulfadiazine: –
Artesunate
• Artesunate is a medication used to treat malaria.
• The intravenous form is preferred to quinidine for severe
malaria.
• Often it is used as part of combination therapy, such as
artesunate plus mefloquine or amodiaquine.
• It is not used for the prevention of malaria
Artemether
• Artemether is used to treat acute uncomplicated malaria.
• It is administered in combination with lumefantrine for
improved efficacy. This combination therapy exerts its effects against the
erythrocytic stages of Plasmodium spp. and may be used to treat infections
caused by P. falciparum and unidentified Plasmodium species.
Atovaquone
• Atovaquone is a naphthoquinone used for the prevention and
treatment of Pneumocystis pneumonia (PCP) and,
• In combination with proguanil, used for prevention and
treatment of P. falciparum malaria
Classification based on MOA
• Antimalarials can be divided into two classes based on
their MOA
• 1) The first class of compounds are characterized by rapid
onset of schizoticidal action
• Includes the cinchona alkaloids, aminoquinolines and
acridines and involves a relatively non-specific mechanism.
• The derivatives in this first group inhibit nucleic acid
and protein synthesis in the protozoal cell.
• Due to the interaction between the drug and DNA.
• The flat aromatic quinoline or acridine ring can position
or intercalate between the base pairs in the DNA-α-helix and the secondary
alcohol group in quinine or the amino groups in the other derivatives provide
secondary binding through hydrogen bond formation.
• Because these events can take place in mammalian host cells
as well as in parasite cells,
• The antimalarial action depends upon selective
accumulation of the drugs in the parasite cell.
• E.g chloroquine, erythrocytic schizonts can concentrate
the drug to a level many times that of the plasma concentration.
• Host cells require a 100-fold greater concentration to be
affected than is necessary to kill parasite cells.
• 2) The second class includes the pyrimidines, biguanides
and sulfones and involves interference with the synthesis of tetrahydrofolic
acid (FH4).
• This mechanism is characterized by a slowly developing
schizonticidal action dependent upon the stage of multiplication of the
parasite.
• The pyrimidine and biguanide derivatives are competitive
inhibitors of dihydrofolic acid (FH2), binding to dihydrofolate reductase and
thereby interfering with conversion of FH2 to FH4. FH4 is necessary
• The effect occurs in host as well as in parasite cells,
but is selective to the parasite because of a greater effective concentration.
• The sulfones as well as sulfonamides interfere with the
synthesis of dihydrofolic acid by competing with p-amino benzoic acid (PABA)
incorporation.
• The metabolites of the sulfones resemble PABA structurally
and when incorporated, produce an inactive coenzyme.
• This mechanism does not operate in mammalian host cells.