MACROLIDES
Contents
• Macrolides – Introduction
• Chemistry of Macrolides
• Over view of Mechanism of action and Resistance of
Macrolides
• Spectrum of Activity of Macrolides
• Study of Individual products
Learning
Objectives
At the end of this lecture, student will be able to
• Discuss the chemistry of Macrolides
• Explain the mode of action and resistance of Macrolides
• Outline the spectrum of activity of Macrolides
• Discuss the structural features of Macrolides and uses
Introduction
• Macrolides are the antibiotics isolated from the
Actinomycetes.
• Picromycin is the first compound of this group identified
as ‘Macrolide’.
• Others in the sequence are erythromycin and carbomycin and
then other macrolides like azithromycin, clarothromycin and oleandomycin.
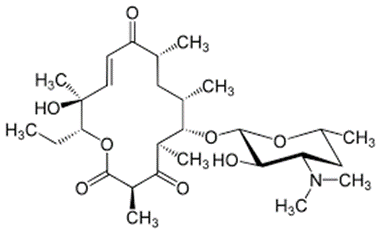
Chemistry
3 common chemical characteristics
• A large lactone ring (i.e., why the name macrolide)
• A ketone group
• A glycosidically linked amino sugar
• The lactone ring has 12, 14 or 16 atoms in it and it is often unsaturated with an olefinic group
conjugated with the ketone function.
• In addition, to the amino sugar, a neutral sugar that is linked glycosidically to the lactone ring may
be present eg., Erythromycin
• They are stable in aqueous solutions at or below room temperature
but are inactivated by acids, bases and heat.
• Macrolides are bases that form salts with pKa values
between 6.0 and 9.0, because of the dimethyl amino group on the sugar moiety.
ERYTHROMYCIN
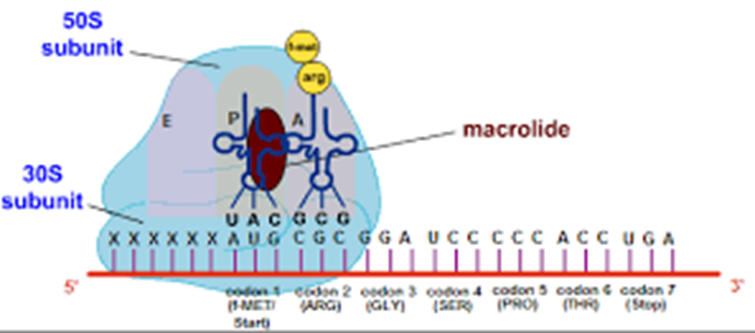
Mechanism
of action
Bacteriostatic
• It binds selectively to specific site on the 50S ribosomal
subunit to prevent the translocation step of bacterial protein synthesis
• It does not bind to mammalian ribosomes and so will not
affect the protein synthesis in mammalian cells
Mechanism
of Resistance
• The nonspecific resistance to the antibacterial action of
erythromycin among many species of Gram-ve bacilli is largely related to the
inability of the antibiotic to penetrate the cell walls of these organisms.
Protoplasts from Gram –Ve bacilli which lack cell walls are sensitive to
erythromycin.
• A highly specific resistance mechanism to the macrolide
antibiotics occurs in erythromycin-resistant
strains of S.aureus. These strains produce an enzyme that methylates a specific
residue at the erythromycin-binding site of the bacterial 50S ribosomal
subunit. The methylated ribisomal RNA remains active in protein synthesis but
no longer binds erythromycin.
Spectrum of
activity
The spectrum of antibacterial activity of the more potent
macrolides, as erythromycin resembles that of penicillin.
They are active against bacterial strains that are resistant
to the penicillins
Effective against most species of Gram+ve bacteria-both
cocci & bacilli, some Gram-ve cocci especially Neisseria species, Treponema
pallidum, etc.
In contrast to penicillins, they are effective against
Mycoplasma, Chlamydia, Campylobacter and Legionella species, some strains of H.
influenzae and Brucella species.
Erythromycin
Isolated from S.erythraceus
• It is a well-tolerated antibiotic
• Used in the treatment of upper respiratory and soft-tissue
infections caused by Gram+ve bacteria.
• Effective against many venereal diseases including
gonorrhea and syphilis
• Useful alternative for the treatment of many infections in
patients allergic to penicillins.
• Erythromycin was shown to be effective therapy for Eaton
agent pneumonia (Mycoplasma pneumoniae), venereal diseases caused by Chlamydia,
bacterial enteritis caused by Campylobacter jejuni, and Legionnaires disease
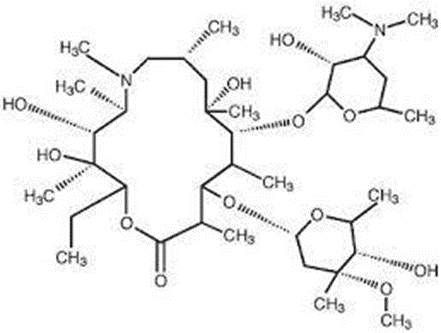
Clarithromycin
• Clarithromycin is well absorbed following oral
administration
• Some of the microbiological properties of clarithromycin
also appear to be superior to those of erythromycin
• It exhibits greater potency against M. pneumoniae,
Legionella spp., Chlamydia pneumoniae, H. influenzae, and M. catarrhalis than
does erythromycin
• Activity against unusual pathogens such as Borrelia
burgdorferi (the cause of Lyme disease) and the Mycobacterium avium complex (MAC)
Azithromycin
• It’s more active against gram-ve organisms (including H.
influenza) than against Gram+ve organisms
• Highly active against intracellular pathogens as
Mycoplasma, Chlamydia, Legionella & Salmonella
• Oral bioavailability is good if administered 1-2 hours
before food as food decreases its absorption
• Its greater activity coupled with its extended half-life
permits a five day dosing schedule for upper respiratory tract infections
• Use: to treat
upper & lower resp. tract infections, skin and soft tissue infections,
urogenital infections including Gonnorrhea.