Anti-Diabetic Drugs

Diabetes mellitus (DM) definition
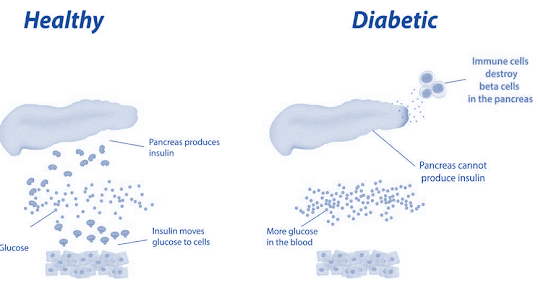
• Diabetes mellitus (DM) It is a metabolic dis- order characterized by hyperglycaemia, glycosuria and ketonaemia.
Pathophysiology of DM
• A widespread pathological change is thickening of capillary basement membrane, increase in vessel wall matrix and cellular proliferation resulting in vascular complications like
• Lumen narrowing,
• Early atherosclerosis,
• Sclerosis of glomerular capillaries,
• Retinopathy,
• Neuropathy
• Peripheral vascular insufficiency.
• Enhanced glycosylation of tissue proteins due to persistent exposure to high glucose concentrations and the accumulation of larger quantities of sorbitol (a reduced product of glucose) in tissues are believed to be causative in the pathological changes of diabetes.
Symptoms of DM
• Frequent Urination
• Blurry Vision
• Tingling in Hand and Feet
• Sudden Weight Loss
• Always Hungry
• Always Thirsty
• Wounds take time to heal
Glycosuria
Types of diabetes mellitus
Type 1 Diabetes
• Type 1 Insulin-dependent diabetes mellitus (IDDM) /juvenile onset diabetes mellitus:
• There is β cell destruction in pancreatic islets; majority of cases are autoimmune (type 1A) antibodies that destroy β cells are detectable in blood.
• In all type 1 cases circulating insulin levels are low and patients are more prone to ketosis.
• This type is less common and has a low degree of genetic predisposition.
Type II (NIDDM)
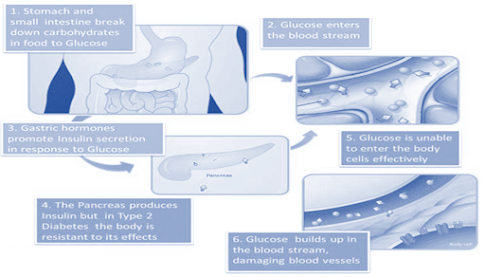
• Type II Noninsulin-dependent diabetes mellitus (NIDDM)/maturity onset diabetes mellitus:
• Has a high degree of genetic predisposition; generally has a late onset (past middle age). Over 90% cases of diabetes are type 2 DM.
• Cause: Reduced sensitivity of peripheral tissues to insulin.
Type 2 Diabetes causes
Anti-Diabetic Drugs
Diabetes management is not a one-size-fits-all approach. Understanding the different types of anti-diabetic drugs is crucial for personalized treatment plans. From insulin to oral medications, each plays a unique role in controlling blood sugar levels.
Insulin
• Insulin was discovered in 1921 by Banting and Best who demonstrated the hypoglycaemic action of an extract of pancreas.
Insulin structure
• Insulin is a two chain polypeptide having 51 amino acids and MW about 6000. The A-chain has 21 while B-chain has 30 amino acids.
• There are minor differences between human, pork and beef insulins: pork insulin is more homologous to human insulin than is beef insulin.
• Insulin is synthesized in the β cells of pancreatic islets
Actions of insulin
• Insulin facilitates glucose transport across cell membrane; skeletal muscle and fat are highly sensitive.
• Insulin facilitates glycogen synthesis from glucose in liver, muscle and fat by stimulating the enzyme glycogen synthase.
• Insulin inhibits gluconeogenesis (from pro- tein, FFA and glycerol) in liver.
• Insulin inhibits lipolysis in adipose tissue and favours triglyceride synthesis.
• In diabetes increased amount of fat is broken down due to unchecked action of lipolytic hormones (glucagon) → increased FFA and glycerol in blood → taken up by liver to produce acetyl-CoA.
• Normally acetyl-CoA is resynthesized to fatty acids and triglycerides, but this process is reduced in diabetics and acetyl CoA is diverted to produce ketone bodies (acetone, acetoacetate,β-hydroxy-butyrate).
• Insulin facilitates AA entry and their synthesis into proteins, as well as inhibits protein breakdown in muscle and most other cells.
Insulin deficiency leads to protein breakdown → AAs are released in blood → taken up by liver and converted to Glucose.
Actions of insulin producing hypoglycaemia
|
Liver |
Muscle |
Adipose |
|
▲Increases glucose uptake and glycogen synthesis |
▲Increases glucose uptake and utilization |
▲Increases glucose uptake and storage as fat and glycogen
|
|
▲Inhibits glycogenolysis and glucose output. |
▲Inhibits proteolysis and release of amino acids. into blood which form the substrate |
▲ Inhibits lipolysis and release of FFA which form substrate for gluconeogenesis in liver |
|
▲Inhibits gluconeogenesis from protein and FFA |
|
|
Fate of insulin
• Insulin is distributed only extracellularly.
• It is a peptide; gets degraded in the g.i.t. if given orally.
• Injected insulin or that released from pancreas is metabolized primarily in liver and to a smaller extent in kidney and muscles.
• The plasma t½ is 5–9 min.
Preparations of insulin
• The older commercial preparations were produced from beef and pork pancreas.
• They contained ~1%(10,000 ppm) of other proteins (proinsulin, other polypeptides, pancreatic proteins, insulin derivatives, etc.) which were potentially antigenic.
• They are no longer produced and have been totally replaced by highly purified pork/beef insulins/recombinant human insulins/insulin analogues.
Highly purified insulin preparations
• In the 1970s improved purification techniques like gel filtration and ion-exchange chromatography were applied to produce ‘monocomponent (MC)’ insulins which contain <10 ppm proinsulin.
• They are less antigenic.
Types of insulin preparations
Regular (soluble) insulin
• It is a buffered neutral pH solution of unmodified insulin stabilized by a small amount of zinc.
• At the concentration of the injectable solution, the insulin molecules self-aggregate to form hexamers around zinc ions.
• After s.c. injection, insulin monomers are released gradually by dilution, so that absorption occurs slowly.
• Peak action is produced only after 2–3 hours and action continues upto 6–8 hours.
Lente insulin (Insulin-zinc suspension)
• Two types of insulin-zinc suspensions have been produced.
• The one with large particles is crystalline and practically insoluble in water (ultralente). It is long-acting.
• The other has smaller particles and is amorphous (semilente), is short- acting.
• Their 7:3 ratio mixture is called ‘Lente insulin’ and is intermediate-acting.
Isophane (Neutral Protamine Hagedorn or NPH) insulin
• Protamine is added in a quantity just sufficient to complex all insulin molecules;
• Neither of the two is present in free form and pH is neutral.
• On s.c. injection, the complex dissociates slowly to yield an intermediate duration of action.
Insulin Preparations available
• 1. Highly purified (monocomponent) pork regular insulin: ACTRAPID MC, RAPIDICA 40U/ml inj.
• 2. Highly purified (MC) pork lente insulin: LENTARD, MONOTARD MC, LENTINSULIN-HPI, ZINULIN 40 U/ml.
• 3. Highly purified (MC) pork isophane (NPH) insulin: INSULATARD 40 U/ml inj.
Human insulins
• In the 1980s, the human insulins (having the same amino acid sequence as human insulin) were produced by recombinant DNA technology in Escherichia coli—‘proinsulin recombinant bacterial’ (prb) and in yeast—‘precursor yeast recombinant’ (pyr), or by ‘enzymatic modification of porcine insulin’ (emp).
Human insulins available
• 1. HUMULIN R: Human regular insulin; 40 U/ml, 100 U/ml.
• 2. HUMULIN-L: Human lente insulin; 40 U/ml, 100 U/ml.
• 3. HUMULIN-N: Human isophane insulin 40 U/ml.
Availability of insulin
• In the USA pork and beef insulins are no longer manufactured, but they are still available in U.K., India and some European countries.
• In Britain now > 90% diabetics who use insulin are taking human insulins or insulin analogues.
• In India also human insulins and analogues are commonly used, except for considerations of cost.
Reactions to insulin
Hypoglycaemia
• can occur in any diabetic following inadvertent injection of large dose, by missing a meal after injection or by performing vigorous exercise.
• The symptoms can be divided into those due to counter-regulatory sympathetic stimulation—sweating, palpitation, tremor;
• And those due to deprivation of the brain of its essential nutrient glucose (neuroglucopenic symptoms)—dizziness, headache, behavioural changes,fatigue.
• Treatment: Glucose must be given orally or i.v. (for severe cases)—reverses the symptoms rapidly.
Local reactions:
• Lipodystrophy of the subcutaneous fat around the injection site may occur if the same site is injected repeatedly. This is rare with the newer preparations.
Allergy:
• This is due to contaminating proteins, and is very rare with human/highly purified insulins. Urticaria, angioedema and anaphylaxis are the manifestations.
Uses of insulin
• Diabetes mellitus
• Insulin is effective in all forms of diabetes mellitus and is a must for type 1 cases, and gestational diabetes.
• Many type 2 cases can be controlled by diet, reduction in body weight and appropriate exercise supplemented, if required, by oral hypoglycaemics.
Insulin requirment in Type 2 DM
• Insulin is needed by patients with type 2 DM patient when:
• Not controlled by diet and exercise or when these are not practicable.
• Primary or secondary failure of oral hypoglycaemics or when these drugs are not tolerated.
• Underweight patients.
• Temporarily to tide over infections, trauma, surgery, pregnancy. In the perioperative period and during labour, monitored i.v. insulin infusion is preferable.
• Any complication of diabetes, e.g. ketoacidosis, nonketotic hyperosmolar coma, gangrene of extremities.
Dose of insulin
• Most type 1 patients require 0.4–0.8 U/kg/day.
• In type 2 patients, insulin dose varies (0.2–1.6U/kg/day) with the severity of diabetes and body weight:
• Obese patients require proportionately higher doses due to relative insulin resistance.
Diabetic ketoacidosis
• Generally occurs in insulin dependent diabetics. (Type 1 DM)
• Precipitating cause is infection; others are trauma, stroke, pancreatitis, stressful conditions and inadequate doses of insulin.
Treatment of DKA
• Insulin
• Intravenous fluids
• KCl: When insulin therapy is instituted ketosis subsides and K+ is driven back intracellularly— dangerous hypokalemia can occur.
• Sodium bicarbonate
• Antibiotics
Hyperosmolar (nonketotic hypergly-caemic) coma
• occurs in type 2 DM.
• Uncontrolled glycosuria of DM produces diuresis resulting in dehydration and haemoconcentration over several days → urine output is finally reduced and glucose accumulates in blood rapidly to > 800 mg/dl, plasma osmolarity is > 350 mOsm/ L → coma, and death can occur
Hyperosmolar coma management
• The general principles of treatment are the same as for ketoacidotic coma, except that faster fluid replacement is to be instituted and alkali is usually not required.
• These patients are prone to thrombosis (due to hyperviscosity and sluggish circulation), prophylactic heparin therapy is recommended.
Oral hypoglycemic drugs
• These drugs lower blood glucose levels and are effective orally.
• The chief drawback of insulin is—it must be given by injection.
Classification of Oral hypoglycemic drugs
Enhance Insulin secretion
1. Sulfonylureas
• First generation: Tolbutamide
• Second generation: Glibenclamide, Glipizide,
2. Meglitinide analogues
• Repaglinide, Nateglinide
3. Glucagon-like peptide-1 (GLP-1) receptor agonists (Injectable drugs)
• Exenatide, Liraglutide
4. Dipeptidyl peptidase-4 (DPP-4) inhibitors
• Sitagliptin, Vildagliptin.
Overcome Insulin resistance
1. Biguanide
• Metformin
2. Thiazolidinediones (PPAR γ activator)
• Pioglitazone
Miscellaneous antidiabetic drugs
• α-Glucosidase inhibitors
• Acarbose, Miglitol, Voglibose
• Sodium-glucose cotransport-2 (SGLT-2) Inhibitor: Dapagliflozin
Drugs which enhance insulin secretion
Sulfonylureas (KATP Channel blockers)
• Mechanism of action: Sulfonylureas provoke a brisk release of insulin from pancreas.
Adverse effects of SU
• Hypoglycaemia
• Hypersensitivity
Meglitinide analogues (KATP Channel blockers)
• MOA: release of insulin from pancreas.
• Drugs: Repaglinide, Nateglinide.
• They induce rapid onset short lasting insulin release.
• Administered before each major meal to control post prandial hyperglycemia.
Glucagon-like peptide-1 (GLP-1) receptor agonists
• GLP-1 is an important incretin released from the gut in response to ingested glucose.
• It induces insulin release from pancreatic β cells, inhibits glucagon release from α cells, slows gastric emptying and suppresses appetite.
• Drugs available: Exenatide, Liraglutide(Victoza)
Dipeptidyl peptidase-4 (DPP-4) inhibitors
• DPP-4 in rapid degradation of endogenous GLP-1, orally active inhibitors of DPP-4 have been developed as indirectly acting insulin secretagogues.
• Sitagliptin, Vildagliptin, Saxagliptin
Drugs which overcome insulin resistance
Biguanide
• Phenformin higher risk of lactic acidosis banned in India since 2003.
• Metformin: Enhances insulin-mediated glucose uptake and disposal in skeletal muscle and fat.
• Adverse effects: Lactic acidosis, g.i. intolerance.
Thiazolidinedione (PPAR γ agonist)
Pioglitazone
• MOA: Glitazones tend to reverse insulin resistance by enhancing GLUT4 expression and translocation.
• Entry of glucose into muscle and fat is improved.
• (Rosiglitazone, is banned in India due to unacceptable increase in risk of myocardial infarction, CHF, stroke and death.)
Miscellaneous
α Glucosidase inhibitors
• Acarbose: inhibits α-glucosidases, the final enzymes for the digestion of carbohydrates in the brush border of small intestine mucosa.
• Flatulence, abdominal discomfort and loose stool are produced in about 50% patients due to fermentation of unabsorbed carbohydrates.
Sodium-glucose co-transport-2 (SGLT-2) Inhibitor
• Practically all the glucose filtered at the glomerulus is reabsorbed in the proximal tubules.
• The major transporter which accomplishes this is SGLT-2,
• Inhibition of SGLT-2 induces glucosuria and lowers blood glucose in type 2 DM, as well as causes weight loss.
Anti-Diabetic Drugs FAQs
- Are Anti-Diabetic Drugs safe for long-term use?
- Anti-diabetic medications are generally safe for long-term use when prescribed and monitored by healthcare professionals.
- Can lifestyle changes alone manage diabetes without medications?
- In some cases, lifestyle changes may be sufficient for diabetes management, but medications are often necessary for optimal control.
- What are the common side effects of anti-diabetic drugs?
- Common side effects include nausea, weight gain, and low blood sugar. However, side effects vary among individuals.
- How often should blood sugar levels be monitored?
- The frequency of monitoring depends on individual circumstances, but regular monitoring is typically recommended.
- Are there alternatives to traditional insulin injections?
- Yes, alternatives such as insulin pumps and inhalable insulin are available, depending on individual needs and preferences.